Embibe Experts Solutions for Chapter: Solid State, Exercise 1: CG PET 2017
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Solid State, Exercise 1: CG PET 2017
Attempt the practice questions on Chapter 9: Solid State, Exercise 1: CG PET 2017 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Solid State, Exercise 1: CG PET 2017 with Hints & Solutions
Carborundum is the commercial name of
How many crystal system are there when and ?
The lattice point per unit cell in the figure
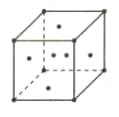
is
A match box exhibits
The cubic unit cell of a metal (molar mass ) has an edge length of . Its density is . The type of unit cell is
The radius of sodium atom isfs . If sodium crystal is a body centred, then the edge length of unit cell is
Number of unit cells present in a cubic shaped ideal crystal of of mass is [Molecular mass ]
Pink colour of non-stoichiometric is due to
