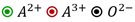Embibe Experts Solutions for Chapter: Solid State, Exercise 1: JEE Main - 6 September 2020 Shift 2
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Solid State, Exercise 1: JEE Main - 6 September 2020 Shift 2
Attempt the free practice questions on Chapter 7: Solid State, Exercise 1: JEE Main - 6 September 2020 Shift 2 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Solid State, Exercise 1: JEE Main - 6 September 2020 Shift 2 with Hints & Solutions
Ionic radii of cation and anion are and respectively. These ions are allowed to crystallize into an ionic solid. This crystal has cubic close packing for is present in all octahedral voids. The edge length of the unit cell of the crystal is___.
When is heated in presence of oxygen, it converts to . The number of correct statement/s from the following is ______ .
A. The equivalent weight of is
B. The number of moles of and in mole of is and respectively.
C. is metal deficient with lattice comprising of cubic closed packed arrangement of ions.
D. The composition of and in is and respectively.
A cubic solid is made up of two elements and . Atoms of are present on every alternate corner and one at the center of cube. is at of the total faces. The empirical formula of the compound is
A metal forms hexagonal close-packed structure. The total number of voids in of it is _____ (Nearest integer)
(Given )
Iron oxide , crystallises in a cubic lattice with a unit cell edge length of . If density of the in the crystal is , then the number of units present per unit cell is _____ (Nearest integer)
Given : Molar mass of and is and respectively.
A sample of a metal oxide has formula . The metal can exist in two oxidation states and . In the sample of , the percentage of metal ions existing in oxidation state is . (nearest integer)
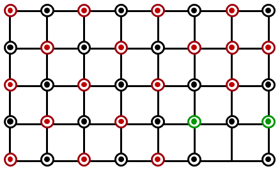
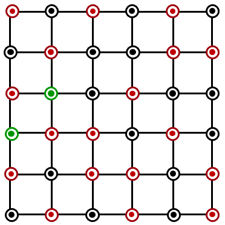
Which of the following represents the lattice structure of containing and ions?
A.
B.
C.
A metal M crystallizes into two lattices :- face centred cubic (fcc) and body centred cubic (bcc) with unit cell edge length of and respectively. The ratio of densities of lattices fcc to bec for the metal M is _______(Nearest integer)