Embibe Experts Solutions for Chapter: Solutions, Exercise 1: JEE Advanced Paper 1 - 2013
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Solutions, Exercise 1: JEE Advanced Paper 1 - 2013
Attempt the free practice questions on Chapter 8: Solutions, Exercise 1: JEE Advanced Paper 1 - 2013 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Solutions, Exercise 1: JEE Advanced Paper 1 - 2013 with Hints & Solutions
The mole fraction of urea in an aqueous urea solution containing of water is . If the density of the solution is , the molarity of urea solution is _______. (Round off the answer to the nearest decimal)
(Given data: Molar masses of urea and water are and , respectively)
Pure water freezes at and pressure. The addition of of ethanol to of water changes the freezing point of the solution. Use the freezing point depression constant of water as . The figures shown below represent the plots of vapour pressure () versus temperature (). [Molecular weight of ethanol is 46 g ]
Among the following, the option representing a change in the freezing point is
Mixture(s) showing positive deviation from Raoult's law at is (are)
On dissolving of a non-volatile non-ionic solute to of benzene, its vapor pressure decreases from to The depression of freezing point of benzene (in ) upon addition of the solute is _______
(Given data: Molar mass and the molal freezing point depression constant of benzene are and respectively)
Give your answer up to three significant figures.
Liquids A and B form ideal solution over the entire range of composition. At temperature , equimolar binary solution of liquids A and B has vapour pressure . At the same temperature, a new solution of A and B having mole fractions , respectively, has vapour pressure of . The value of , in the new solution is ____.
(Given that the vapour pressure of pure liquid A is at temperature )
The plot given below shows curves (where, is the pressure and is the temperature) for two solvents and and isomolal solutions of in these solvents. completely dissociates in both the solvents.
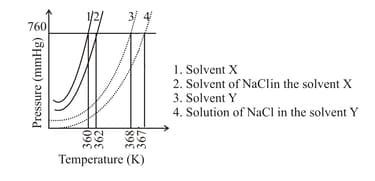
On the addition of equal number of moles of a non-volatile solute in equal amount (in ) of these solvents, the elevation of boiling point of solvent is three times that of solvent . The solute is known to undergo dimerization in these solvents. If the degree of dimerization is in the solvent , the degree of dimerization in the solvent is _______.
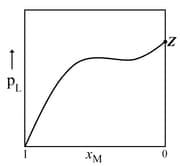
For a solution formed by mixing liquids and , the vapour pressure of plotted against the mole fraction of in solution is shown in the following figure, Here, and represent mole fractions of and , respectively, in the solution. The correct statement(s) applicable to this system is(are) -
Benzene and naphthalene form an ideal solution at room temperature. For this process, the true statement(s) is (are)
