Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: JEE Advanced Paper 1 - 2017
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: JEE Advanced Paper 1 - 2017
Attempt the free practice questions on Chapter 4: Thermodynamics, Exercise 1: JEE Advanced Paper 1 - 2017 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: JEE Advanced Paper 1 - 2017 with Hints & Solutions
One mole of an ideal gas at , undergoes two reversible processes, followed by , as shown below. If the work done by the gas in the two processes are same, the value of is
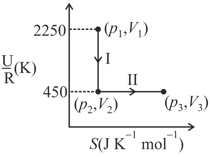
( : internal energy, entropy, pressure, volume, : gas constant)
(Given: molar heat capacity at constant volume, of the gas is )
Give your answer as the nearest integer.
Tin is obtained from cassiterite by reduction with coke. Use the data given below to determine the minimum temperature (in ) at which the reduction of cassiterite by coke would take place.
Assume that the enthalpies and the entropies are temperature independent.
For a reaction, , the plots of with time at temperatures are given below.
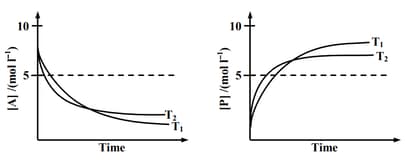
If , the correct statement(s) is (are)
(Assume are independent of temperature and ratio of is greater than . Here H, S , G and K are enthalpy, entropy, Gibbs energy and equilibrium constant, respectively.)
The surface of copper gets tarnished by the formation of copper oxide. gas was passed to prevent the oxide formation during heating of copper at 1250 K. However, the gas contains 1 mole % of water vapour as impurity. The water vapour oxidises copper as per the reaction given below:
Is the minimum partial pressure of (in bar) needed to prevent the oxidation at . The magnitude of value of is ____.
(Given: total pressure = 1 bar, R (universal gas constant) are mutually immiscible.
At
)
Round off the answer up to the nearest integer.
An ideal gas undergoes a reversible isothermal expansion from state to state followed by a reversible adiabatic expansion from state to state The correct plot representing the changes from state to state is(are)
(: pressure, : volume, : temperature, : enthalpy, : entropy)
Choose the reaction(s) from the following options, for which the standard enthalpy of reaction is equal to the standard enthalpy of formation.
A reversible cyclic process for an ideal gas is shown below. Here, are pressure, volume and temperature, respectively. The thermodynamic parameters are heat, work, enthalpy and internal energy, respectively.

The correct option(s) is (are)
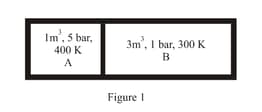
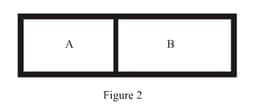
A closed tank has two compartments A and B, both filled with oxygen (assumed to be ideal gas). The partition separating the two compartments is fixed and is a perfect heat insulator (Figure). If the old partition is replaced by a new partition which can slide and conduct heat but does not allow the gas to leak across (Figure ), the volume (in ) of the compartment A after the system attains equilibrium is . Write the value of .