Embibe Experts Solutions for Chapter: Atoms and Nuclei, Exercise 1: JEE Main - 12th January 2019 Shift 2
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Atoms and Nuclei, Exercise 1: JEE Main - 12th January 2019 Shift 2
Attempt the free practice questions on Chapter 21: Atoms and Nuclei, Exercise 1: JEE Main - 12th January 2019 Shift 2 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR PHYSICS solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Atoms and Nuclei, Exercise 1: JEE Main - 12th January 2019 Shift 2 with Hints & Solutions
A radioactive nucleus decays by two different process. The half life of the first process is minutes and that of the second process is . The effective half-life of the nucleus is calculated to be . The value of is ______.
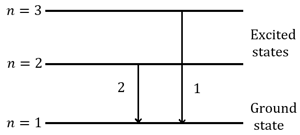
For hydrogen atom, and are the wavelengths corresponding to the transitions and respectively as shown in figure. The ratio of and is . The value of is ______.
The radius of electron's second stationary orbit in Bohr's atom is . The radius of orbit will be
If the binding energy of ground state electron in a hydrogen atom is , then, the energy required to remove the electron from the second excited state of will be: . The value of is _____.
The mass of proton, neutron and helium nucleus are respectively . The binding energy of helium nucleus is:
A light of energy is incident on a hydrogen atom in its ground state. The atom absorbs the radiation and reaches to one of its excited states. The angular momentum of the atom in the excited state is . The value of is ______ (use )
An electron of a hydrogen like atom, having , jumps from energy state to energy state, The energy released in this process, will be: (Given )
Where Rydberg
constant Speed of light in vacuum
Planck's constant
Nucleus having and equal number of protons and neutrons has binding energy per nucleon. Another nucleus of has total nucleons and binding energy per nucleons. The difference of binding energy of and will be ______ .
