Embibe Experts Solutions for Exercise 1: SAT 2020
Embibe Experts Scholastic Aptitude Test (SAT) Solutions for Exercise - Embibe Experts Solutions for Exercise 1: SAT 2020
Attempt the free practice questions from Exercise 1: SAT 2020 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR SAT solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 1: SAT 2020 with Hints & Solutions
Reaction of organic compound ‘A’ with ‘B’ in acidic condition gives compound ‘C’. Compound ‘B’ reacts with alkaline solution and gives compound ‘A’. Compound ‘C’ gives compound ‘B’ as one of the products when reacted with sodium hydroxide solution. Which of the following statements is/are correct?
I. ‘A’ is
II. ‘B’ is
III. ‘C’ is
IV. ‘A’ is sweet smelling substance
The compound when treated with alkaline potassium permanganate gives , and with concentrated sulphuric acid gives and . The compounds and are respectively:
An organic compound A on heating with concentrated gave product B and on warming with alkaline gave compound C. Compound A on heating with compound C in presence of concentrated formed compound D, which has fruity smell. Identify the compounds A, B, C and D.
Two organic compounds ' ' and ' ' react with sodium metal and both produce the same gas ' ', but with sodium hydrogen carbonate only compound reacts to give a gas ' ’. Identify ' ', ' ', ' ' and ' ':
On oxidation with alkaline followed by acidification of the reaction mixture, which one of the following alcohols would produce an acid having three structural isomers?
Detergents are also called surface-active agents (surfactants). These have two distinct parts: one hydrophilic spherical part and another hydrophobic long tail made of carbons chain. Two experiment ‘A’ and ‘B’ were carried out. In experiment ‘A’, surfactant was added in a beaker containing water. In experiment ‘B’, surfactant was added in a beaker containing hexane.
Following are possible results in these experiments.
I. In experiment ‘A’(see figure) ‘a’ micelle is formed, where hydrophobic part is inside the micelle and hydrophilic part is outside the micelle.
II. In experiment ‘B’(see figure ‘b’) micelle of reverse type is formed where hydrophilic part is inside the micelle and hydrophobic part is outside the micelle.
III. Micelle of reverse type is formed in experiment ‘A’.
IV. Micelles are large enough to scatter light in ‘A’
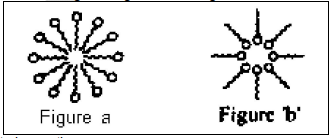
Correct observations are:
Which of the following options containing formula, bonding and nature of aqueous solution respectively is correct for the compound formed by two elements A and B having atomic numbers 1 and 17, respectively?
