Embibe Experts Solutions for Exercise 1: SAT 2019
Embibe Experts Scholastic Aptitude Test (SAT) Solutions for Exercise - Embibe Experts Solutions for Exercise 1: SAT 2019
Attempt the free practice questions from Exercise 1: SAT 2019 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR SAT solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 1: SAT 2019 with Hints & Solutions
A student was performing an experiment to understand the enzyme-substrate reaction. The student measured the formation of coloured product using a colorimeter. The student plotted the graph below which shows the reaction rate versus the substrate concentration.
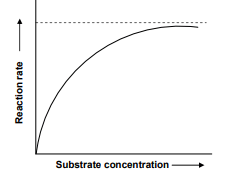
Following interpretations were drawn by the student:
A. The higher concentration of substrate acts as an enzyme inhibitor.
B. It is sigmoidal curve with sharp transition from low to high reactions rates over the increasing substrate concentration.
C. The curve reaches a plateau and does not further increase with increasing substrate concentrations due to saturation of enzyme with the substrate.
Choose which of the interpretations of the graph are correct.
The values of stoichiometric coefficients and in the following reaction after balancing are, respectively:
Match the chemical reaction given in the list- with type of chemical reactions given in the list- and select the correct answer from the options given below:
| List- (Chemical reactions) | List- (Type of chemical reactions) |
| Addition | |
| Elimination | |
| Redox | |
| Substitution |
Which of the following statements are true?
I. On heating the kinetic energy of particles in solids does not change because they have a fixed position.
II. Sublimation is the change of gaseous state directly to solid state without going through liquid state and vice versa.
III. The movement of particles from an area of higher concentration to lower concentration is called diffusion.
IV. The rate of evaporation is not affected by increasing the temperature.
