Embibe Experts Solutions for Exercise 1: SAT 2019
Embibe Experts Scholastic Aptitude Test (SAT) Solutions for Exercise - Embibe Experts Solutions for Exercise 1: SAT 2019
Attempt the free practice questions from Exercise 1: SAT 2019 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR SAT solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 1: SAT 2019 with Hints & Solutions
Read the following statements.
Statement I: Sodium metal reacts violently with water to produce heat and fire.
Statement II: Potassium metal reacts violently with water to form potassium hydroxide and hydrogen gas.
Select the correct answer from the option given below
Which of the following set of reactions will NOT occur?
I.
II.
III.
IV.
The following observations are given for four metals:
I. Metal H does not react with dilute .
II. Metal K reacts with warm water.
III. Metal L does not react with water but displaces metal H from its aqueous salt solution.
IV. Metal M reacts with cold water.
Choose the correct decreasing order of reactivity of these metals amongst the following.
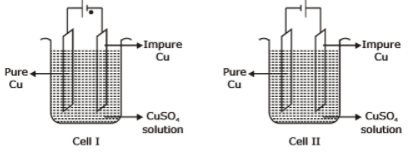
Consider the electrochemical cells(I and II) shown in the following figures and select the correct statement about these cells
Read the following table:
| Metal | Reaction with | ||
| solution | solution | solution | |
| X | No reaction | No reaction | No reaction |
| Y | No reaction | No reaction | Displacement reaction |
| Z | Displacement reaction | Displacement reaction | Displacement reaction |
Based on the above table consider the following statements
I. Reaction of Y with solution produces metal.
II. Z is the most reactive element and X is the least reactive.
III. Y is more reactive than X and less reactive than Z.
IV. Metal Y produces on reaction with solution.
Which of the following options gives the correct statements?
Two beakers A and B contain iron(II) sulphate solution. In the beakers A and B, small pieces of copper and zinc are placed respectively. It is found that a grey deposit forms on the zinc but not on the copper. From these observations, it can be concluded that:
