Embibe Experts Solutions for Chapter: Metals and Non-Metals, Exercise 1: Jharkhand Board-2019
Embibe Experts Science Solutions for Exercise - Embibe Experts Solutions for Chapter: Metals and Non-Metals, Exercise 1: Jharkhand Board-2019
Attempt the practice questions on Chapter 3: Metals and Non-Metals, Exercise 1: Jharkhand Board-2019 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR SCIENCE solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Metals and Non-Metals, Exercise 1: Jharkhand Board-2019 with Hints & Solutions
Write the name of the main ore of mercury metal.
What are amphoteric oxides?
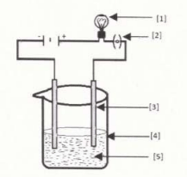
In the given figure, the purification of copper in electric decomposition is shown. Label against and .
What are amphoteric oxides? Give an example.
Name a non-metal which exists in the liquid state.
Why is sodium kept immersed in kerosene oil?
Show the formation of by the transfer of electrons.
Compound and aluminium are used to join railway tracks.
(i) Identify the compound .
(ii) Name the reaction.
(iii) Write down its chemical equation.
