Embibe Experts Solutions for Exercise 1: EXERCISE
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Exercise 1: EXERCISE
Attempt the free practice questions from Exercise 1: EXERCISE with hints and solutions to strengthen your understanding. Gamma Question Bank for Engineering Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 1: EXERCISE with Hints & Solutions
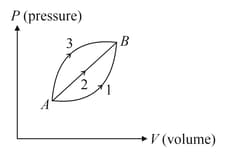
A given mass of ideal gas changes its state from to along three different paths and If and be the heat supplied to the gas along respective paths then
For a given amount of ideal gas, if be the slope of curve for its isothermal process and be the slope of curve for its adiabatic process then equals
Two moles of an ideal gas undergoes adiabatic process and changes its state from to . The change in internal energy of the gas will be
At a constant pressure, a given amount of ideal diatomic gas expands under normal condition. The heat supplied to the gas is Then
A mixture of gases contains moles of oxygen and moles of argon at absolute temperature The total internal energy of this system (neglecting vibrations) is universal gas constant ]
Two samples A and B of a gas initially at the same temperature and pressure, are compressed from the volume, to ( isothermally and, adiabatically). The final pressure
A gas is suddenly compressed to th of its original volume. Rise in temperature of the gas if initial temperature is , will be (given
An ideal gas is heated at constant pressure and absorbs amount of heat. If the ratio then
