Exercise
Embibe Experts Physics Solutions for Exercise
Simple step-by-step solutions to Exercise questions of ATOMS from Physics Crash Course (Based on Revised Syllabus - 2023). Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Exercise with Hints & Solutions
Write two limitations of Bohr's atomic model.
What are the limitations of Bohr's model
Calculate the ratio of the frequencies of the radiation emitted due to the transition of the electron in a hydrogen atom from its second permitted energy level to the first level to the highest permitted energy level to the second permitted level.
The wavelength of the second line of the Balmer series in the hydrogen spectrum is . Calculate the wavelength of the first line.
The largest wavelength in the ultraviolet region of hydrogen spectrum is . The smallest wavelength in the infrared region of the hydrogen spectrum (to the nearest integer) is
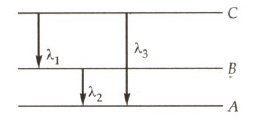
Which of the relation given in options gives the relation between the three wavelengths from the energy level diagram shown below?
According to the classical electromagnetic theory velocity of electron moving around a proton in hydrogen atom in an orbit of radius is . Calculate the initial frequency of the light emitted by the electron revolving around a proton in hydrogen atom.
As per Bohr model, the minimum energy (in ) required to remove an electron from the ground state of doubly ionized atom is
