Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 2: Level 2
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 2: Level 2
Attempt the practice questions on Chapter 8: Thermodynamics, Exercise 2: Level 2 with hints and solutions to strengthen your understanding. Physics Crash Course NEET solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 2: Level 2 with Hints & Solutions
and are specific heats at constant pressure and constant volume, respectively. It is observed that for hydrogen gas, for nitrogen gas. The correct relation between and is
A gas at state changes to state through path and shown in figure. The change in internal energy is and , respectively. Then
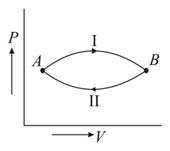
If system is in thermal equilibrium with and is separately in thermal equilibrium with then and are in thermal equilibrium. From which thermodynamics law, does this follow?
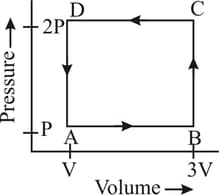
A thermodynamic system is taken through the cycle as shown in the figure. Heat rejected by the gas during the cycle is:
The efficiency of Carnot engine is . It rejects of heat to sink. The work done by the engine is
In an adiabatic process, the state of a gas is changed from to . Which of the following relation is correct?
In a Carnot engine, when heat is taken by a perfect gas from the source, then the temperature of the source
The source and sink temperatures of a Carnot engine are and , its efficiency is
