Embibe Experts Solutions for Chapter: Gaseous State, Exercise 1: EXERCISE
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Gaseous State, Exercise 1: EXERCISE
Attempt the free practice questions on Chapter 4: Gaseous State, Exercise 1: EXERCISE with hints and solutions to strengthen your understanding. Practice Book for KVPY Aptitude Test - Stream SA Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Gaseous State, Exercise 1: EXERCISE with Hints & Solutions
To an evacuated vessel with movable piston under external pressure of of and of an unknown compound (vapour pressure at ) are introduced. Considering the ideal gas behaviour, determine the total volume (in litre) of the gases at .
At , two balloons of equal volume and porosity are filled to a pressure of . One is with and other is with of . The balloon leaks to a pressure of in hour. How long will it take for balloon to reach a pressure of ?
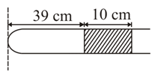
A tube of length is containing a gas in two sections separated by a mercury column of length as shown in figure. The open end of tube is just inside the surface in container. Find the pressure of gas in two sections. [assume atmospheric pressure of ]
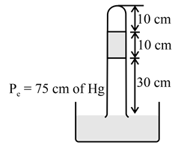
Which of the lines in the figure reflect correctly on the log scale, the temperature dependent of the root mean square velocity of the molecules.
In a basal metabolism measurement timed at , a patient exhaled of air measured over water at . The vapour pressure of water at is . The barometric pressure was . The exhaled air analyzed volume oxygen and the inhaled air volume oxygen. Both on a dry basis neglecting any solubility of the gases in water and any difference in the total volumes of inhaled and exhaled air, calculate the rate of oxygen consumption by the patient in () per minute.
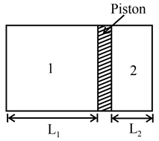
The closed cylinder shown in the figure has a freely moving piston separating chambers and . Chamber contains of gas, and chamber contains of helium gas. When equilibrium is established, what will be the ratio ? What is the ratio of the number of moles of to the number of moles of ? (Molecular weights of and are and ).
The density of a mixture of and at NTP is . Calculate the partial pressure of .
Given a one meter long glass tube closed at one end having a uniform cross-section containing a mercury column of length, at a distance of from the closed end. By what distance would this column move down, if the tube is held vertically with the open end downwards. [Take atmospheric pressure to be of ]