Embibe Experts Solutions for Chapter: Gaseous State, Exercise 2: KVPY PROBLEMS (PREVIOUS YEARS)
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Gaseous State, Exercise 2: KVPY PROBLEMS (PREVIOUS YEARS)
Attempt the practice questions on Chapter 4: Gaseous State, Exercise 2: KVPY PROBLEMS (PREVIOUS YEARS) with hints and solutions to strengthen your understanding. Practice Book for KVPY Aptitude Test - Stream SA Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Gaseous State, Exercise 2: KVPY PROBLEMS (PREVIOUS YEARS) with Hints & Solutions
The ratio of the root-mean-square velocity of hydrogen at to that of nitrogen at is the closest to:
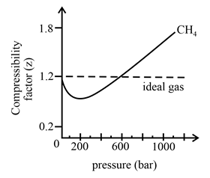
In the following compressibility factor vs. pressure graph at , the compressibility of at pressures deviates from ideal behaviour because:
According to Graham's Law, the rate of diffusion of and follows the order:
In a closed vessel, an ideal gas at is heated from to . The final pressure of the gas will approximately be:
A reaction has an activation energy of . The rate increases -fold when the temperature is increased from to . The temperature is closest to:
[Gas constant, ]
A mixture of toluene and benzene boils at . Assuming ideal behaviour, the mole fraction of toluene in the mixture is closest to:
[Vapour pressures of pure toluene and pure benzene at are and , respectively. ]
The pressure volume isotherm of a Van der Waals gas, at the temperature at which it undergoes gas to liquid transition, is correctly represented by:
A container of volume can withstand a maximum pressure of at before exploding. The maximum amount of nitrogen (in ) that can be safely put in this container at this temperature is closest to:
