Embibe Experts Solutions for Chapter: Chemical Reactions and Equations, Exercise 1: Exercise
Embibe Experts Science Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Reactions and Equations, Exercise 1: Exercise
Attempt the free practice questions on Chapter 1: Chemical Reactions and Equations, Exercise 1: Exercise with hints and solutions to strengthen your understanding. THINK ABOVE AND BEYOND SCIENCE PRACTICE BOOKS solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Reactions and Equations, Exercise 1: Exercise with Hints & Solutions
Ms. Shakuntala is demonstrating a science experiment to her students. She is trying to explain the effect of oxygen on metals. Sanjana and Asif were helping the teacher. They measured the mass of a piece of iron wool (fine strands of iron) as . Then the teacher started experimenting and used tongs to hold the iron in a Bunsen flame.
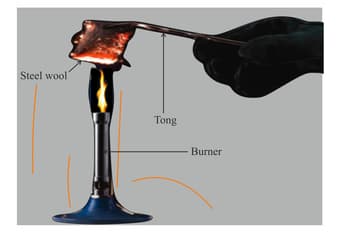
The teacher and the students noticed it turned darker and dull. She let it cool and then measured the mass again. It was now . Describe what happened to the mass of the iron when it was heated.

The gas X is a major component of air, and it does not support combustion. Gas X is a colourless, odourless, tasteless gas that is the most plentiful element in Earth's atmosphere and is a constituent of all living matter. It is important for plant growth and can be 'fixed' by lightning or added to soils by means of fertilisers. The liquid state of this gas is used as a coolant for computers and in medicine to remove unwanted skin, warts and pre-cancerous cells. It is also used in the food industry, for example, a bag of chips is flushed with the above gas X.
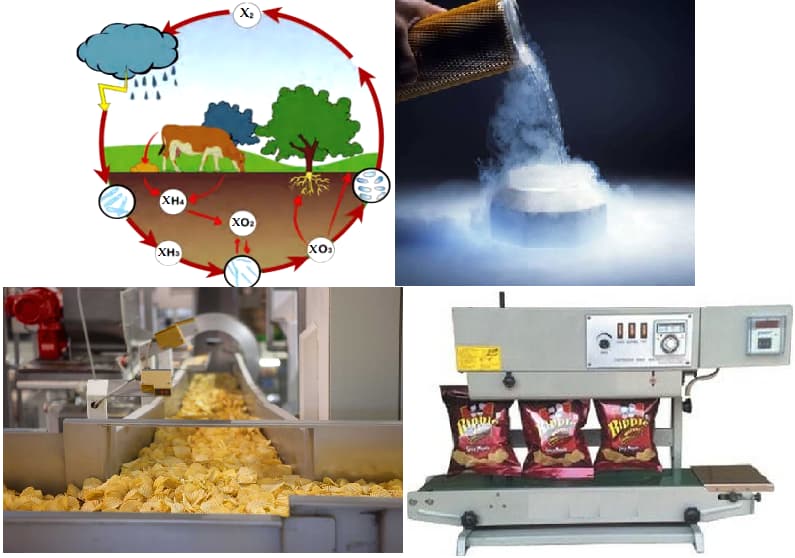
From the given comprehension, can you guess the name of the gas X and write the reason why the bags of chips are flushed with this gas?
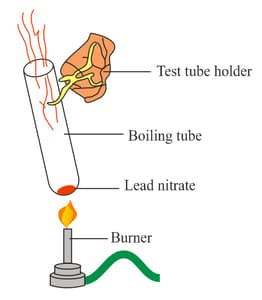
Rahul is a very curious student who frequently visits the lab in his free time. Rahul was interested in conducting an experiment, and he let the teacher know about the same. The teacher gave him a test tube containing lead nitrate and instructed him to heat it up and watch what happened. He began heating the test tube, and after a while, brown-coloured vapours were coming out. The teacher then asked Rahul to analyse the reaction taking place. Could you assist Rahul in identifying the type of the reaction and its chemical equation? Also, help Rahul to identify the gas evolved as brown vapours.
Omkar and his older brother Abhi were studying late at night. They went to the kitchen in the middle of the study and brought some potato chips for eating. They observed that some odour was emanating from the chips, and the taste was also unusual. Abhi, a 10th-grade student, was familiar with the process that has caused the foul smell and bad taste to the potato chips and explained the same to his younger brother. Can you describe the process that Abhi must have explained to Omkar?

Four groups of students in Mrs Kiran's class were investigating how the mass of magnesium changes when it burns. Burning is a phenomenon where a substance reacts with oxygen. They noted that it burns with a white sparkling flame similar to the sparks we see when we burn crackers.
At the end of the experiment, they noted the initial mass of magnesium before burning and the mass of magnesium at the end of burning. These data are shown below in the table.
| Group | Mass of magnesium in the starting | Mass of magnesium at the end |
- Three groups analysed the increase in the mass of the metal at the end of the reaction. Could you state the reason for this increase? Also, mention whether the reaction is exothermic or endothermic.
- Which group has not performed the experiment correctly?
- Which law of chemical reaction can be explained with the help of this experiment? What other data is needed to prove the law correctly.
Mrs Brown’s class were investigating how the mass of magnesium changes when it burns.
At the end of the experiment, they collected the results together in a table.
| Group | Mass of magnesium in the starting | Mass of magnesium at the end |
- Explain which group’s result was unexpected. Give a reason for your answer.
When a black metal compound XO is heated with a colourless gas Y2, then metal X and another compound Y2O are formed. Metal X is red-brown in colour which does not react with dilute acids at all. Gas Y2 can be prepared by the action of a dilute acid on any active metal. The compound Y2O is a liquid at room temperature which can turn anhydrous copper sulphate blue.
(a) What do you think is metal X?
(b) What could be gas Y2?
(c) What is compound XO?
(d) What is compound Y2O?
(e) Write the chemical equation of the reaction which takes place on heating XO with Y2.
(f) What type of chemical reaction is illustrated in the above equation?
Anthracite contains carbon and generally has the highest heating value of all ranks of coal. It burns to give heat and light, thus, it is used as a fuel. This heat energy is widely used in power generation and in steel industries. Coal is used for household purposes since early times. Taking the ideal condition for coal that it is pure carbon with no impurities, such a coal when burnt will liberate carbon dioxide gas along with heat energy.

- If coal is represented by a chemical symbol , write the reaction for the complete combustion of coal.
- Could you explain the type of reaction given in part one?
- Can this reaction be considered as an oxidation reaction? If yes, explain why?
