Embibe Experts Solutions for Chapter: Matter in Our Surroundings, Exercise 1: Exercise
Embibe Experts Science Solutions for Exercise - Embibe Experts Solutions for Chapter: Matter in Our Surroundings, Exercise 1: Exercise
Attempt the practice questions on Chapter 1: Matter in Our Surroundings, Exercise 1: Exercise with hints and solutions to strengthen your understanding. THINK ABOVE AND BEYOND SCIENCE PRACTICE BOOKS solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Matter in Our Surroundings, Exercise 1: Exercise with Hints & Solutions
Aqueous lead (II) nitrate and aqueous potassium iodide are added to a dish containing water as shown.
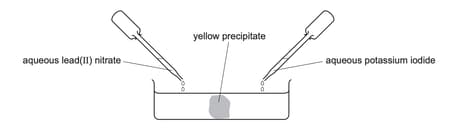
A yellow precipitate forms after a few minutes.
Which process occurs before the precipitate forms?
The diagrams show the arrangement of particles in three different physical states of substance .
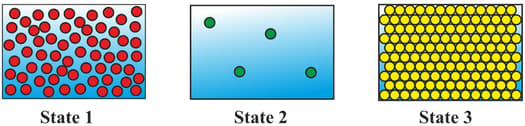
Which statement about the physical states of substance is correct?
When potassium permanganate is put in water, the colorless water attains purple color. What could be the possible reasons?
(i) The particles of water and potassium permanganate are in motion.
(ii) The process of diffusion is taking place.
(iii) The process of sublimation is taking place.
(iv) Only the particles of potassium permanganate are moving
The diagram shows a cup of tea

Which row describes the water particles in the air above the cup compared with the water particles in the cup?
| Moving faster | Closer together | |
| A |  |
 |
| B |  |
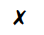 |
| C | 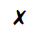 |
 |
| D | 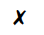 |
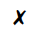 |
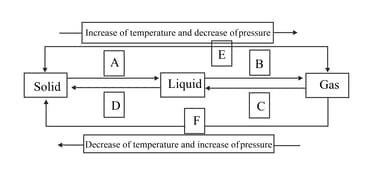
The symbols A, B, C, D, E and F in the following diagram represent various types of processes for the change of states of matter, namely solid, liquid and gas. Name the processes and then redraw the complete scheme.
A student heats a beaker containing ice and water. He measures the temperature of the beaker as a function of time and draws the following graph.
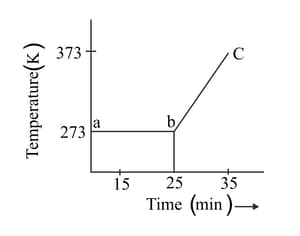
At what temperature and time ice melts?
A student heats a beaker containing ice and water. He measures the temperature of the beaker as a function of time and draws the following graph.
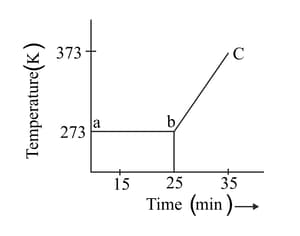
What does the curve bc represent?
Look at the diagram below. Jar A contains a red-brown gas whereas jar B contains a colourless gas. The two gas jars are separated by a glass plate placed between them. After some time when the glass plate is removed it is seen that jar A and jar B turns reddish-brown.
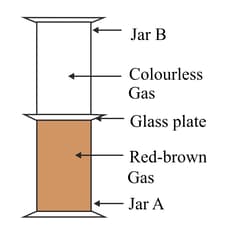
Name one coloured solid and one colourless liquid which can show the same phenomenon.
