Structure of Matter
Important Questions on Structure of Matter
In the following table a represents a shared electron pair. Let us find out the similarities among the various entries in the table and have a grasp of the whole thing.
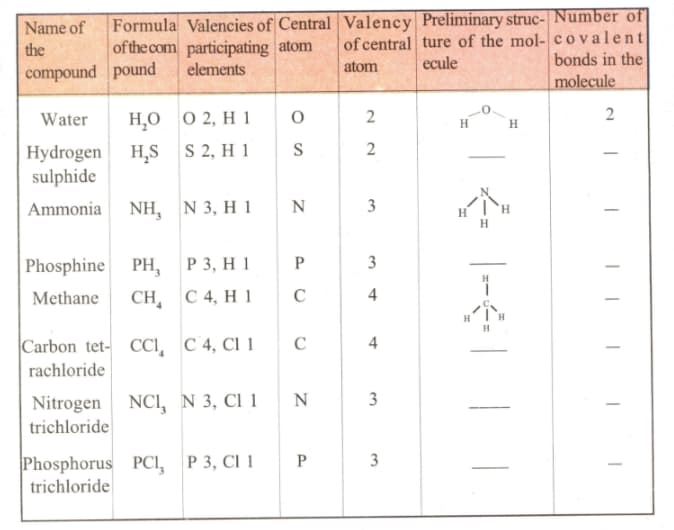
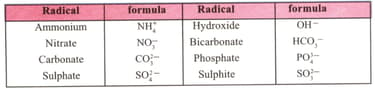
Construct the formulae of the compounds using above table.
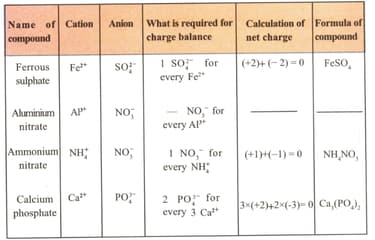
Fill up the blank spaces in the following table.
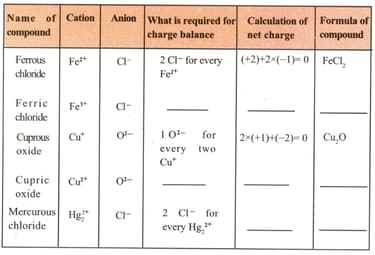
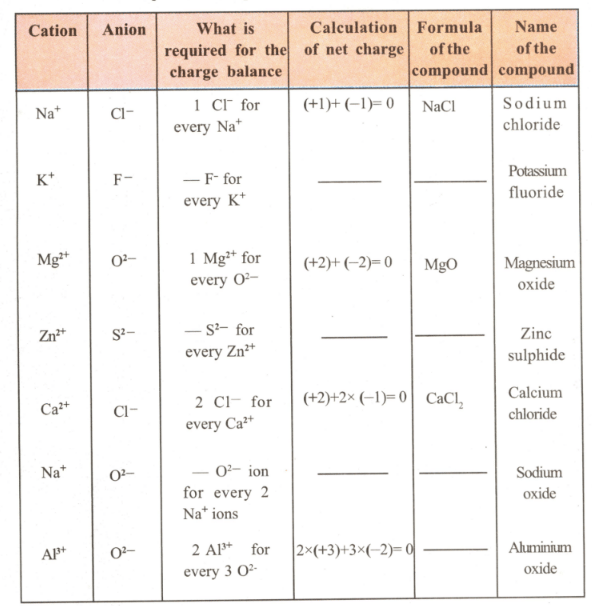
Fill up the blank spaces in the following table.
A gold atom is heavier than an iron atom; of which element - gold or iron - will contain a greater number of atoms?
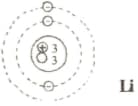
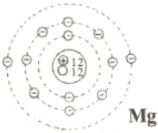
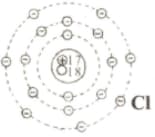
In the following table, diagrams of the atoms of lithium, sodium, magnesium and chlorine are shown. Complete the table with information regarding the quantities mentioned in it.
| Atom | Proton | Electron | Neutron | Mass number | Atomic number | Symbol of the element |
|
|
||||||
|
|
||||||
|
|
||||||
|
|
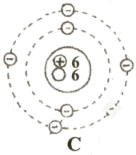
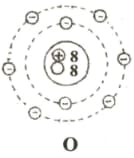
In this diagram, 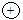 ,
, 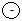 and
and  represents proton, electron and neutron respectively. Find the atomic numbers and mass numbers of carbon and oxygen. How will you express this through symbols?
represents proton, electron and neutron respectively. Find the atomic numbers and mass numbers of carbon and oxygen. How will you express this through symbols?