General Periodic Trends
Important Questions on General Periodic Trends
Which of the following statements does not apply to elements belonging to the same period of the periodic table?
In the Modern Periodic Table, calcium (atomic number 20) is surrounded by elements with atomic numbers 12,19,21, and 38. Which of these have physical and chemical properties resembling calcium?
Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the Periodic Table. Write the electronic configuration of these two elements. Which of these will be more electronegative? Why?
Which of the following statements is an incorrect statement about the trends when going from left to right across the periods of the periodic table?
Table given below shows a part of the periodic table.
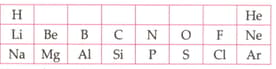
Using this table explains why flourine is more reactive than chlorine.
Table given below shows a part of the periodic table.

Using this table explain why the atomic size of Mg is less than that of Na.
Taking an example of an element of the third period, discuss the trend of reactivity from left to right.
In this section, each question has two matching lists. Choices for the correct combination of elements from List-I and List-II are given as options (a), (b), (c) and (d) out of which one is correct?
| List-I | List-II |
| (P) Metallic character in a group | 1. Decreases |
| (Q) Valency in a period | 2. First increases then decreases |
| (R) Valence electrons in a group | 3. Remain same |
| (S) Atomic size in a period | 4. Increases |
In the periodic table, the ionisation potential in a group _____ from top to bottom.
Which of the following statements is correct about element and element ?
Which is true about electronegativity order?
Among O, C, F, Cl, Br, the correct order of increasing atomic radii is
Arrange the following elements in order of their increasing ionisation energies :
Listed below are the locations of certain elements in groups and periods of the periodic table. Arrange these elements in the expected order of increasing first ionisation energy.
P : Element in the fourth period and group IVA
Q : Element in the third period and group VIA
R : Element in the sixth period and group IIIA
S : Element in the second period and group VIA
Observe the following trends in the periodic properties, in the periodic table.
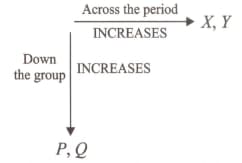
Identify the properties X, Y, P and Q respectively.
Which of the following increases along the period?
The atoms of elements belonging to the same group of periodic table have the same number of
The element with the smallest size in group is
The chemistry of lithium is very similar to that of magnesium even though they are placed in different groups because
All the elements in a period in the periodic table have the same

