Chemical Properties of Acids
Important Questions on Chemical Properties of Acids
How is the concentration of hydronium ions affected when a solution of an acid is diluted?
While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Daivik, a class 10 student studied the reaction between a carbonate and an acid in the lab. His results are shown in the given graph: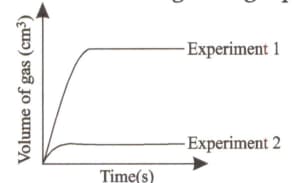
Which of the following experimental conditions did he use?
State the basicity of phosphorous acid.
Write any three chemical properties of acids.
What is observed when hot concentrated sulphuric acid is added to sulphur?
What is observed when dilute sulphuric acid is added to solid sodium carbonate?
Name the gas evolved when dilute reacts with sodium hydrogen carbonate. How is it recognised?
Which gas is evolved when metal carbonates or metal hydrogen carbonate reacts with dilute acids?
Breaking apart of the positive and negative ions of a compound in solution is called
One molecule of aluminium hydroxide will require how many molecules of dil. for complete neutralisation?
Here (X) is
Acidity in the sugarcane juice is removed by adding
A student takes some zinc granules in a test tube and adds dilute hydrochloric acid to it. He would observe that the colour of the zinc granules changes to
Which gas is evolved when acids react with metal carbonates?
When black copper oxide placed in a beaker is treated with dilute , its colour changes to
Equal lengths of of magnesium ribbons are taken in test tubes and . Hydrochloric acid is added to test tube , while acetic acid is added to test tube . In which test tube will the fizzing occur more vigorously and why?
Write the word equation and balanced equation for the reaction taking place when dilute hydrochloric acid reacts with iron filings.
Write the word equation and balanced equation for the reaction taking place when dilute sulphuric acid reacts with aluminium powder.
Write the word equation and balanced equation for the reaction taking place when dilute hydrochloric acid reacts with magnesium ribbon.

