G L Mittal and TARUN MITTAL Solutions for Chapter: Internal Energy : First Law of Thermodynamics : Specific Heat, Exercise 1: QUESTIONS
G L Mittal Physics Solutions for Exercise - G L Mittal and TARUN MITTAL Solutions for Chapter: Internal Energy : First Law of Thermodynamics : Specific Heat, Exercise 1: QUESTIONS
Attempt the practice questions on Chapter 21: Internal Energy : First Law of Thermodynamics : Specific Heat, Exercise 1: QUESTIONS with hints and solutions to strengthen your understanding. ISC Physics Class XI Part 1 solutions are prepared by Experienced Embibe Experts.
Questions from G L Mittal and TARUN MITTAL Solutions for Chapter: Internal Energy : First Law of Thermodynamics : Specific Heat, Exercise 1: QUESTIONS with Hints & Solutions
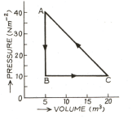
The adjoining diagram shows the pressure-volume graph of thermodynamic processes of an ideal gas. Determine the work done in processes separately and the work done in the complete cycle
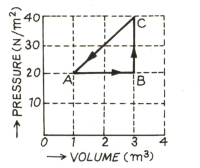
In the adjoining diagram are shown the changes taking place in a thermodynamic system in going from the initial state to the states and and finally returning to the state If joule, joule and the heat spent in the change from state to is joule, then determine work done by the system in the change from to .
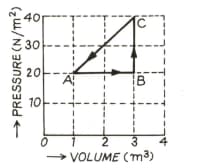
In the adjoining diagram are shown the changes taking place in a thermodynamic system in going from the initial state to the states and and finally returning to the state If joule, joule, and the heat spent in the change from state to is joule, then determine heat released from the system in the change from to .
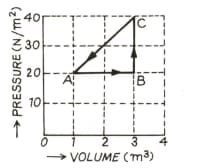
In the adjoining diagram are shown the changes taking place in a thermodynamic system in going from the initial state to the states and and finally returning to the state If joule, joule and the heat spent in the change from state to is joule, then determine the value of .
The initial pressure and volume of a gas are respectively. They are increased to In which process will more work have to be done : (1) first increasing the volume only and increasing the pressure, or (2) first increasing the pressure only and then increasing the volume? Will the change in internal energy of the gas in these process be different?
Why is internal energy said to be a unique function?
Prove that the difference in the molar specific heats of an ideal gas is nearly Given :
What is meant by 'degrees of freedom' of a gas molecule?
