Embibe Experts Solutions for Exercise 1: EXERCISE
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Exercise 1: EXERCISE
Attempt the free practice questions from Exercise 1: EXERCISE with hints and solutions to strengthen your understanding. Gamma Question Bank for Engineering Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 1: EXERCISE with Hints & Solutions
Figure shows diagram for a given mass of an ideal gas for the process During this process, density of the gas is
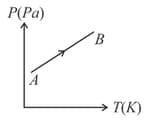
Figure shows curves for a given mass of an ideal gas at pressures and Then
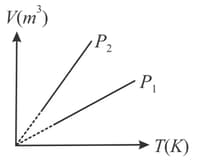
The diagram of a gas at constant temperature are drawn. The curve is for a constant mass and temperature and curve is for a constant mass and temperature Select the correct alternative.

At the ratio of density of fixed mass of an ideal gas divided by its pressure is The ratio at is
A gas is filled in a cylinder. Its temperature is increased by and volume decreases by If pressure remains constant, what percentage of mass of the gas leaks out ?
At a temperature the pressure of argon contained in a bulb is The bulb is put in a bath having temperature higher by then the first one. Now, of argon has to be removed to maintain original pressure. The temperature is equal to
Consider an ideal gas contained in a vessel. If the intermolecular interactions suddenly begins to acts, which of the following will happen?
