Gary Horner Solutions for Chapter: Form, Exercise 14: Summative assessment
Gary Horner Chemistry Solutions for Exercise - Gary Horner Solutions for Chapter: Form, Exercise 14: Summative assessment
Attempt the practice questions on Chapter 6: Form, Exercise 14: Summative assessment with hints and solutions to strengthen your understanding. MYP Chemistry A concept-based approach Years 4&5 solutions are prepared by Experienced Embibe Experts.
Questions from Gary Horner Solutions for Chapter: Form, Exercise 14: Summative assessment with Hints & Solutions
The graph illustrates the changes in state for myristic acid, a saturated fatty acid, as it is heated. Saturated acids have been linked to high levels of cholesterol in humans and an increases chance of coronary heart disease.
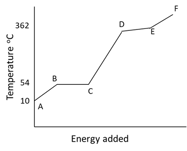
From your analysis of the graph, explain what is happening between and .
The graph illustrates the changes in state for myristic acid, a saturated fatty acid, as it is heated. Saturated acids have been linked to high levels of cholesterol in humans and an increases chance of coronary heart disease.
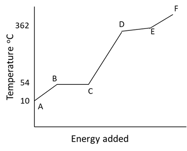
State the name used to describe the process at region at and .
The graph illustrates the changes in state for myristic acid, a saturated fatty acid, as it is heated. Saturated acids have been linked to high levels of cholesterol in humans and an increases chance of coronary heart disease.
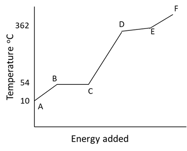
Describe what is happening to the particles in this pure substance between and .
The graph illustrates the changes in state for myristic acid, a saturated fatty acid, as it is heated. Saturated acids have been linked to high levels of cholesterol in humans and an increases chance of coronary heart disease.
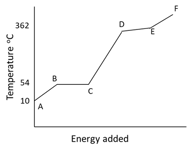
What is the boiling point of this liquid?
The graph illustrates the changes in state for myristic acid, a saturated fatty acid, as it is heated. Saturated acids have been linked to high levels of cholesterol in humans and an increases chance of coronary heart disease.
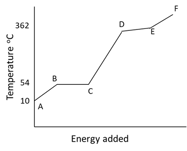
The state of matter for this substance between and is a gas. Explain what will happen to the particles and the and the state of matter if the energy is removed from the system?
All of the salts are soluble in water. Design a series of tests to identify the composition of salts . Your method should include a description of apparatus used and the descriptions of the experimental techniques.
Design a results table to accompany your experimental design.
As a result of high humidity in the chemical storeroom, the labels on five separate reagent bottles were lost. You will need to design a series of analytical tests to identify their composition.

Identify the composition of the unknown salts and .
