Gary Horner Solutions for Chapter: Movement, Exercise 12: Demonstration
Gary Horner Chemistry Solutions for Exercise - Gary Horner Solutions for Chapter: Movement, Exercise 12: Demonstration
Attempt the practice questions on Chapter 10: Movement, Exercise 12: Demonstration with hints and solutions to strengthen your understanding. MYP Chemistry A concept-based approach Years 4&5 solutions are prepared by Experienced Embibe Experts.
Questions from Gary Horner Solutions for Chapter: Movement, Exercise 12: Demonstration with Hints & Solutions
Demonstration
Electrolysis of molten zinc chloride
Safety
• This demonstration should be performed in a fume hood.
• Wear safety glasses.
• Follow correct disposal procedures.
Materials
• Large porcelain crucible
• of zinc chloride
• Tripod stand and clay-pipe triangle
• Bunsen burner
• Retort stand and clamp
• 2 carbon electrodes
• Connecting wires and crocodile clips
• 1 light bulb
• DC power supply
Method
1. Set up the apparatus as shown in the diagram.
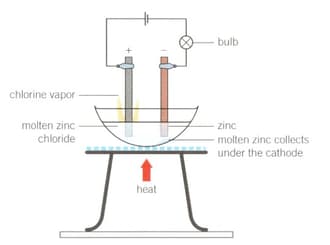
2. Light the Bunsen burner, open the gas sleeve to produce a blue roaring flame and heat continuously until a molten solution of zinc chloride is produced.
What did you observe about the light bulb as the zinc chloride solid began to melt and become molten? Support your answer with scientific reasoning.
Demonstration
Electrolysis of molten zinc chloride
Safety
• This demonstration should be performed in a fume hood.
• Wear safety glasses.
• Follow correct disposal procedures.
Materials
• Large porcelain crucible
• of zinc chloride
• Tripod stand and clay-pipe triangle
• Bunsen burner
• Retort stand and clamp
• 2 carbon electrodes
• Connecting wires and crocodile clips
• 1 light bulb
• DC power supply
Method
1. Set up the apparatus as shown in the diagram.
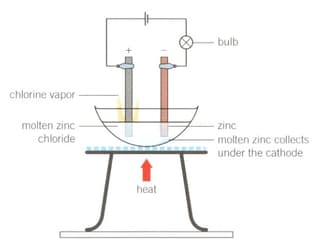
2. Light the Bunsen burner, open the gas sleeve to produce a blue roaring flame and heat continuously until a molten solution of zinc chloride is produced.
Construct balanced half-equations for the reactions occurring at the anode and the cathode, and the overall chemical equation.
