I E Irodov Solutions for Chapter: THERMODYNAMICS AND MOLECULAR PHYSICS, Exercise 4: THE SECOND LAW OF THERMODYNAMICS, ENTROPY
I E Irodov Physics Solutions for Exercise - I E Irodov Solutions for Chapter: THERMODYNAMICS AND MOLECULAR PHYSICS, Exercise 4: THE SECOND LAW OF THERMODYNAMICS, ENTROPY
Attempt the practice questions on Chapter 2: THERMODYNAMICS AND MOLECULAR PHYSICS, Exercise 4: THE SECOND LAW OF THERMODYNAMICS, ENTROPY with hints and solutions to strengthen your understanding. Problems in General Physics solutions are prepared by Experienced Embibe Experts.
Questions from I E Irodov Solutions for Chapter: THERMODYNAMICS AND MOLECULAR PHYSICS, Exercise 4: THE SECOND LAW OF THERMODYNAMICS, ENTROPY with Hints & Solutions
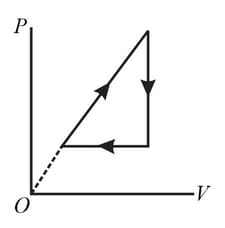
An ideal gas goes through a cycle, consisting of
(a) isochoric, adiabatic and isothermal lines,
(b) isobaric, adiabatic and isothermal lines,
with the exception that, the isothermal process proceeds at the maximum temperature of the whole cycle.An ideal gas goes through a cycle consisting of isothermal, polytropic and adiabatic lines, with the isothermal process proceeding at the maximum temperature of the whole cycle. Find the efficiency of such a cycle, if the absolute temperature varies -fold within the cycle.
An ideal gas, with the adiabatic exponent , goes through a direct (clockwise) cycle, consisting of adiabatic, isobaric and isochoric lines. Find the efficiency of the cycle, if in the adiabatic process, the volume of the ideal gas
(a) increases -fold,
(b) decreases -fold.
Calculate the efficiency of a cycle consisting of isothermal, isobaric and isochoric lines, if in the isothermal process, the volume of the ideal gas with the adiabatic exponent ,
(a) increases -fold,
(b) decreases -fold.
Find the efficiency of a cycle consisting of two isochoric and two isothermal lines, if the volume varies -fold and the absolute temperature -fold within the cycle. The working substance is an ideal gas with the adiabatic exponent .
Find the efficiency of a cycle, consisting of two isobaric and two isothermal lines, if the pressure varies -fold and the absolute temperature -fold within the cycle. The working substance is an ideal gas with the adiabatic exponent .
An ideal gas with the adiabatic exponent , goes through a cycle within which the absolute temperature varies -fold. Find the efficiency of this cycle.
A weightless piston divides a thermally insulated cylinder into two equal parts. One part contains one of an ideal gas, with adiabatic exponent , the other is evacuated. The initial gas temperature is . The piston is released and the gas fills the whole volume of the cylinder. Then, the piston is slowly displaced back to the initial position. Find the increment of the internal energy and the entropy of the gas, resulting from these two processes.
