Equivalence of Work and Heat
Important Questions on Equivalence of Work and Heat
The adjoining diagram shows the pressure-volume graph of thermodynamic processes of an ideal gas. Determine the work done in processes separately and the work done in the complete cycle
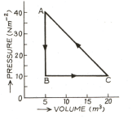
The figure show a graph of a gas. Calculate the work done.
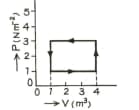
The figure show a graph of a gas. Calculate the work done.
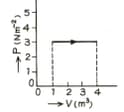
.
The figure show a graph of a gas. Calculate the work done.

The figure show a graph of a gas. Calculate the work done.
Show that for diatomic gas, the ratio of the two specific heats is
A hot piece of iron is immersed in cold water. Has the internal energy of water increased? Has any work been done by the iron?
A piece of lead is hammered. Does the internal energy of the lead increase? Does the heat enter the lead from outside?
Can the temperature of a gas increased, keeping its pressure and volume constant?
The temperature of a gas rises during an adiabatic compression, although no heat is given to the gas from outside. Explain.
Write down the formula of mechanical work equivalent of heat and explain the symbol used.

