K K Sharma Solutions for Chapter: Thermodynamics, Exercise 1: TOPICWISE QUESTIONS
K K Sharma Physics Solutions for Exercise - K K Sharma Solutions for Chapter: Thermodynamics, Exercise 1: TOPICWISE QUESTIONS
Attempt the practice questions on Chapter 3: Thermodynamics, Exercise 1: TOPICWISE QUESTIONS with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Waves and Thermodynamics NEET solutions are prepared by Experienced Embibe Experts.
Questions from K K Sharma Solutions for Chapter: Thermodynamics, Exercise 1: TOPICWISE QUESTIONS with Hints & Solutions
One mole of an ideal gas at an initial temperature of kelvin does joules of work adiabatically. If the ratio of specific heats of this gas at constant pressure and at constant volume is , then the final temperature of the gas will be
The volume of a ideal gas at is . It expands adiabatically to the volume . If , then the work done is
A gas for which is heated at constant pressure. The percentage of total heat given that will be used for external work is
Find the amount of work done to increase the temperature of one mole of an ideal gas by if it is expanded adiabatically under the condition .
Two samples, and having same composition and initially at the same temperature and pressure are compressed from a volume to , isothermally andadiabatically. The final pressure of is
Select the wrong statement for the isothermal expansion of an ideal gas.
A sample of an ideal gas initially having internal energy , is allowed to expand adiabatically performing work Now heat is supplied to the gas to bring it to the initial pressure, keeping volume constant. If final internal energy is , then is,
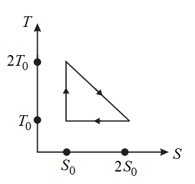
The - diagram of a reversible engine cycle is given in the figure. Its efficiency is,