Kerala Board Solutions for Chapter: Structure of Atom, Exercise 2: Let us assess
Kerala Board Chemistry Solutions for Exercise - Kerala Board Solutions for Chapter: Structure of Atom, Exercise 2: Let us assess
Attempt the free practice questions on Chapter 1: Structure of Atom, Exercise 2: Let us assess with hints and solutions to strengthen your understanding. Chemistry Standard IX Part 1 solutions are prepared by Experienced Embibe Experts.
Questions from Kerala Board Solutions for Chapter: Structure of Atom, Exercise 2: Let us assess with Hints & Solutions
The mass number of an atom is . The M shell of this atom contains electrons; draw the Bohr model of the atom.
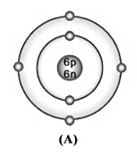
Bohr model of atom A is given (symbols are not real);
Write the atomic number, mass number, and electronic configuration of the atoms.
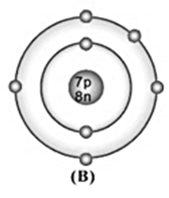
Bohr model of atom B is given (symbols are not real);
Write the atomic number, mass number, and electronic configuration of the atoms.
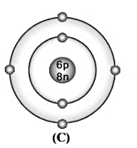
Bohr model of atom C is given (symbols are not real);
Write the atomic number, mass number, and electronic configuration of the atoms.
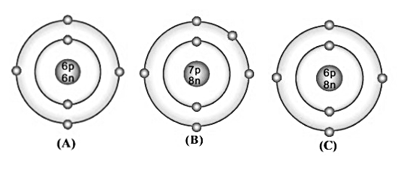
Bohr model of atom A, B, C is given (symbols are not real);
Among these, which are isotopes? Why?
Symbols (not real) of some atoms are given,
Find The atomic number and mass number of these elements.
Symbols (not real) of some atoms are given,
Which among these are isotopic pairs?
Symbols (not real) of some atoms are given,
Draw the Bohr model of atom Q.
