Kerala Board Solutions for Chapter: Reactivity Series and Electrochemistry, Exercise 1: Exercise
Kerala Board Chemistry Solutions for Exercise - Kerala Board Solutions for Chapter: Reactivity Series and Electrochemistry, Exercise 1: Exercise
Attempt the practice questions on Chapter 3: Reactivity Series and Electrochemistry, Exercise 1: Exercise with hints and solutions to strengthen your understanding. Chemistry Standard X Part 1 solutions are prepared by Experienced Embibe Experts.
Questions from Kerala Board Solutions for Chapter: Reactivity Series and Electrochemistry, Exercise 1: Exercise with Hints & Solutions
Sodium chloride in solid state is not an electrical conductor because its ions have no freedom of movement. But electricity flows through molten sodium chloride. When sodium chloride melts, the positively charged sodium ions and the negatively charged chloride ions are free to move.
Which ion is attracted to the positive electrode (anode)?
Sodium chloride in solid state is not an electrical conductor because its ions have no freedom of movement. But electricity flows through molten sodium chloride. When sodium chloride melts, the positively charged sodium ions and the negatively charged chloride ions are free to move.
What is the chemical reaction taking place at the anode?
Sodium chloride in solid state is not an electrical conductor because its ions have no freedom of movement. But electricity flows through molten sodium chloride. When sodium chloride melts, the positively charged sodium ions and the negatively charged chloride ions are free to move.
Which is the gas liberated at the anode?
Sodium chloride in solid state is not an electrical conductor because its ions have no freedom of movement. But electricity flows through molten sodium chloride. When sodium chloride melts, the positively charged sodium ions and the negatively charged chloride ions are free to move.
Which ion is attracted to the negative electrode (cathode)? Write the change happening to it.
Sodium chloride in solid state is not an electrical conductor because its ions have no freedom of movement. But electricity flows through molten sodium chloride. When sodium chloride melts, the positively charged sodium ions and the negatively charged chloride ions are free to move.
Ions like and are present in a solution of sodium chloride.
Which are the ions attracted to the positive electrode?
Ions like and are present in a solution of sodium chloride.
Which are the ions attracted to the negative electrode?
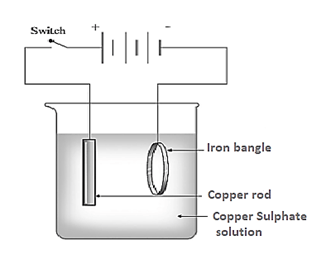
Observe the picture given. This is the process of electroplating of copper on iron bangle.
Which solution is used as electrolyte?