Kerala Board Solutions for Chapter: Reactivity Series and Electrochemistry, Exercise 2: Extended Activities
Kerala Board Chemistry Solutions for Exercise - Kerala Board Solutions for Chapter: Reactivity Series and Electrochemistry, Exercise 2: Extended Activities
Attempt the practice questions on Chapter 3: Reactivity Series and Electrochemistry, Exercise 2: Extended Activities with hints and solutions to strengthen your understanding. Chemistry Standard X Part 1 solutions are prepared by Experienced Embibe Experts.
Questions from Kerala Board Solutions for Chapter: Reactivity Series and Electrochemistry, Exercise 2: Extended Activities with Hints & Solutions
Compare the electrolysis of molten potassium chloride and solution of potassium chloride. What are the processes taking place at the cathode and the anode?
You are given a solution of , a solution of , a rod and a ribbon. How can you arrange a galvanic cell using these? Write down the reactions taking place at the cathode and the anode.
Keep two carbon rods immersed in a copper sulphate solution. Then pass the electricity through the same solution.
At which electrode does colour change occur- (anode or cathode)
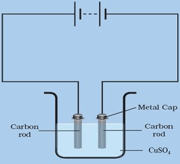
Keep two carbon rods immersed in a copper sulphate solution. Then pass the electricity through the same solution.
Is there any colour change in blue colour of the copper sulphate solution?
When electricity is passed through the solution of copper sulphate which contains carbon rods as electrodes, the colour change is observed at cathode.
Write down the chemical equations for the changes occurring here.
When acidified copper sulphate solution is electrolysed, oxygen is obtained at anode. What arrangements are to be made for this? Find the element deposited at the cathode.
When Galvanic cells are made using metals like and . What will be nature of reactions in each cell?
(Reactivity: )
How many Galvanic cells can be made by using the metals .
