Lawrie Ryan and Roger Norris Solutions for Chapter: Enthalpy Changes, Exercise 8: Question
Author:Lawrie Ryan & Roger Norris
Lawrie Ryan Chemistry Solutions for Exercise - Lawrie Ryan and Roger Norris Solutions for Chapter: Enthalpy Changes, Exercise 8: Question
Attempt the free practice questions on Chapter 6: Enthalpy Changes, Exercise 8: Question with hints and solutions to strengthen your understanding. Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years) solutions are prepared by Experienced Embibe Experts.
Questions from Lawrie Ryan and Roger Norris Solutions for Chapter: Enthalpy Changes, Exercise 8: Question with Hints & Solutions
EASY
AS and A Level
IMPORTANT
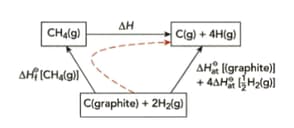
Use the information in Figure and the information below to demonstrate that the average bond energy of the bond is .