Lawrie Ryan and Roger Norris Solutions for Chapter: Equilibria, Exercise 16: Questions
Lawrie Ryan Chemistry Solutions for Exercise - Lawrie Ryan and Roger Norris Solutions for Chapter: Equilibria, Exercise 16: Questions
Attempt the free practice questions on Chapter 8: Equilibria, Exercise 16: Questions with hints and solutions to strengthen your understanding. Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years) solutions are prepared by Experienced Embibe Experts.
Questions from Lawrie Ryan and Roger Norris Solutions for Chapter: Equilibria, Exercise 16: Questions with Hints & Solutions
Suggest a suitable indicator to find the end-points of the reactions between: nitric acid and aqueous ammonia.
Suggest a suitable indicator to find the end-points of the reactions between: aqueous sodium hydroxide and sulphuric acid.
Suggest a suitable indicator to find the end-points of the reactions between aqueous potassium hydroxide and butanoic acid.
Suggest why phenolphthalein would not be a suitable indicator to use to find the end-point when hydrochloric acid is titrated against urea, a weak base.
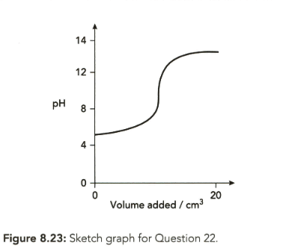
The sketch graph (Figure ) shows the change in when an acid and an alkali react with one being added slowly to the other. Both acid and alkali have a concentration . Which one of these statements is correct?