M K Verma Solutions for Chapter: Chemical Equilibrium, Exercise 1: TOPICWISE QUESTIONS
M K Verma Chemistry Solutions for Exercise - M K Verma Solutions for Chapter: Chemical Equilibrium, Exercise 1: TOPICWISE QUESTIONS
Attempt the free practice questions on Chapter 5: Chemical Equilibrium, Exercise 1: TOPICWISE QUESTIONS with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry 1 NEET solutions are prepared by Experienced Embibe Experts.
Questions from M K Verma Solutions for Chapter: Chemical Equilibrium, Exercise 1: TOPICWISE QUESTIONS with Hints & Solutions
Consider the reaction, , in a closed container at equilibrium. At a fixed temperature, what will be the effect of adding more on the equilibrium concentration of
An example of reversible reaction is
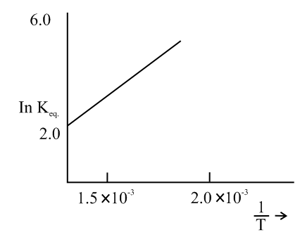
The graph given below relates for a reaction. The reaction must be
The degree of dissociation of , obeying the equilibrium, , is approximately related to the pressure at equilibrium by
If pressure is applied to the equilibrium of . The melting point of the solid
Van't Hoff's equation giving the effect of temperature on chemical equilibrium is represented as
Equilibrium constant at is and at it is . Then the ratio of activation energy will be
For the reaction and
Given
If the degree of dissociation of and are same, then the total pressure at equilibrium of and are in the ratio of:
