Umakant Kondapure, Collin Fernandes, Nipun Bhatia, Vikram Bathula and, Ketki Deshpande Solutions for Chapter: Kinetic Theory of Gases and Radiations, Exercise 2: Critical Thinking
Umakant Kondapure Physics Solutions for Exercise - Umakant Kondapure, Collin Fernandes, Nipun Bhatia, Vikram Bathula and, Ketki Deshpande Solutions for Chapter: Kinetic Theory of Gases and Radiations, Exercise 2: Critical Thinking
Attempt the practice questions on Chapter 3: Kinetic Theory of Gases and Radiations, Exercise 2: Critical Thinking with hints and solutions to strengthen your understanding. MHT-CET TRIUMPH Physics Multiple Choice Questions Part - 2 Based on Std. XI & XII Syllabus of MHT-CET solutions are prepared by Experienced Embibe Experts.
Questions from Umakant Kondapure, Collin Fernandes, Nipun Bhatia, Vikram Bathula and, Ketki Deshpande Solutions for Chapter: Kinetic Theory of Gases and Radiations, Exercise 2: Critical Thinking with Hints & Solutions
In an isothermal reversible expansion, if the volume of of oxygen at $27^{\circ} \mathrm{C}$ is increased from to , then the work done by the gas will be
Efficiency of a Carnot engine is $50 \%$ when temperature of outlet is $500 \mathrm{K}$. In order to increase efficiency up to $60 \%$ keeping temperature of intake the same, what is temperature of outlet?
The volume of air increases by $5 \%$ in its adiabatic expansion. The percentage decrease in its pressure will be
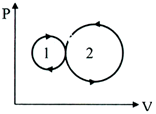
In the following indicator diagram, the net amount of work done will be
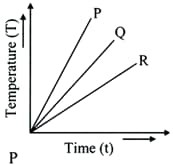
The temperature v/s time graph is shown .Out of $\mathrm{P}, \mathrm{Q}$ or $\mathrm{R}$ Which of the substances has the lowest specific heat?
A gas at absolute temperature of has pressure of . Boltzmann's constant The number of molecules per is of the order of,
Assertion: If a gas is heated isothermally, then no part of the heat supplied is used to increase the internal energy.
Reason: Change in internal energy equals work done on the system.
A Carnot engine whose efficiency is takes in heat from a source maintained at a temperature of . It is desired to have an engine of efficiency . Then the intake temperature for the same exhaust (sink) temperature must be,
