How Can We Represent the Organisation of Electrons in an Atom?
Important Questions on How Can We Represent the Organisation of Electrons in an Atom?
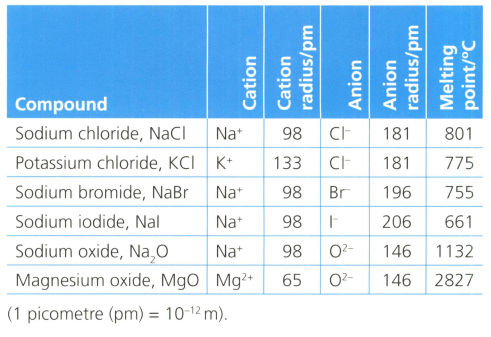
Analyse and evaluate the data about ionic compounds that are listed in the table below.
Identify and make scientifically supported judgments to explain any relationships between the size (radius) of ions present, and the predicted properties of their compounds. Your explanation should refer to your knowledge of ions, ionic bonding and the behaviour of electric charges. State any assumptions in your explanation.
The first electron affinity is the energy change when one mole of gaseous atoms gains a mole of electrons. The table below shows the first electron affinity for selected atoms.
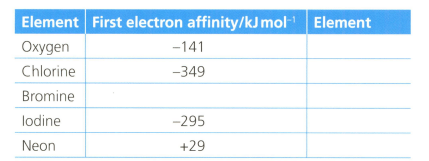
In terms of electrostatics, oxygen has a lower first electron affinity than chlorine.
Explain why neon has a positive value of electron affinity.
The first electron affinity is the energy change when one mole of gaseous atoms gains a mole of electrons. The table below shows the first electron affinity for selected atoms.
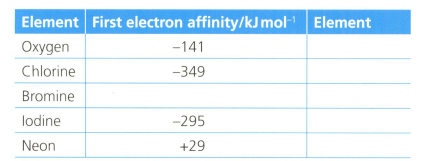
Explain why, in terms of electrostatics, oxygen has a lower first electron affinity than chlorine.
The first electron affinity is the energy change when one mole of gaseous atoms gains a mole of electrons. The table below shows the first electron affinity for selected atoms.
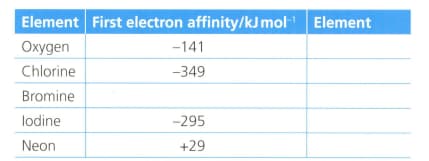
The equation for the first electron affinity of oxygen is .
Formulate an equation (with state symbols) for the second electron affinity of oxygen.
The first electron affinity is the energy change when one mole of gaseous atoms gains a mole of electrons. The table below shows the first electron affinity for selected atoms.
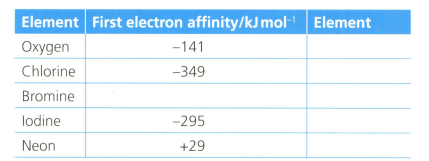
Explain the meaning of the following sentence: "the first electron affinity of oxygen is ."
The first electron affinity is the energy change when one mole of gaseous atoms gains a mole of electrons. The table below shows the first electron affinity for selected atoms.
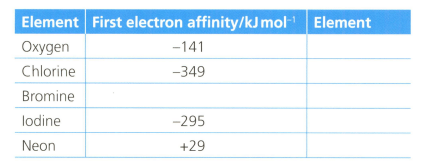
Explain the reason for the relative sizes of the values of the electron affinity for all the elements.
You travel to an alternate universe where the atomic orbits are different from those on Earth, but all other aspects of the atoms are the same. In this universe, you find that the first (the lowest energy) shell is filled with three electrons and the second shell can hold a maximum of nine electrons. You discover an element Z that has five electrons in its atom.
Would you expect Z to be more likely to form a cation or an anion? Suggest a possible charge on this ion.
You are provided with eight cards of unknown elements (below), Suggest how they should be sorted in a logical sequence across a period based on your understanding of their physical and chemical properties.
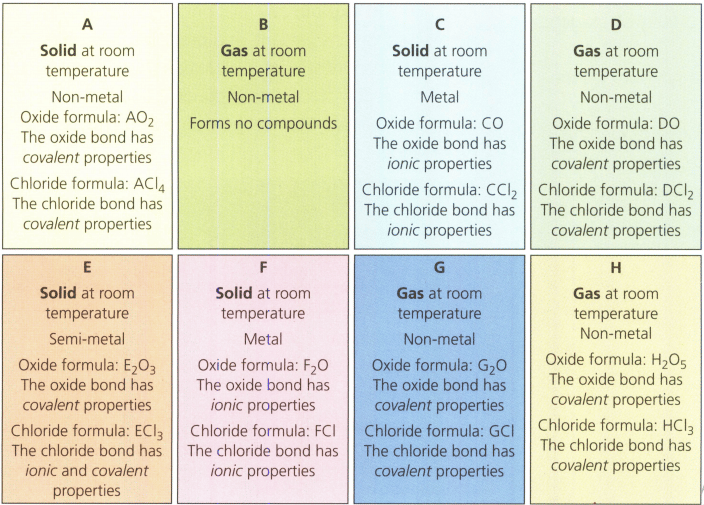
State electron arrangements of the main energy levels (shells) of atoms of a magnesium ion.
State electron arrangements of the main energy levels (shells) of atoms of a nitride ion.
How can we represent the organisation of electrons in an atom?

