Gary Horner Solutions for Chapter: Balance, Exercise 14: Data-based question: Industrial reaction conditions for the production of ammonia
Gary Horner Chemistry Solutions for Exercise - Gary Horner Solutions for Chapter: Balance, Exercise 14: Data-based question: Industrial reaction conditions for the production of ammonia
Attempt the practice questions on Chapter 1: Balance, Exercise 14: Data-based question: Industrial reaction conditions for the production of ammonia with hints and solutions to strengthen your understanding. MYP Chemistry A concept-based approach Years 4&5 solutions are prepared by Experienced Embibe Experts.
Questions from Gary Horner Solutions for Chapter: Balance, Exercise 14: Data-based question: Industrial reaction conditions for the production of ammonia with Hints & Solutions
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
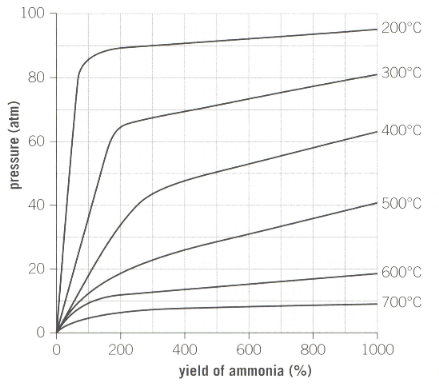
What temperature achieves the highest yield of ammonia?
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
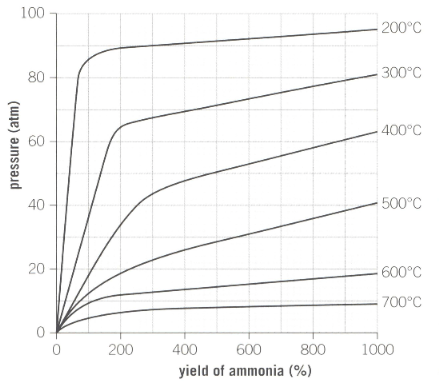
Describe how the yield changes as the pressure is increased.
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
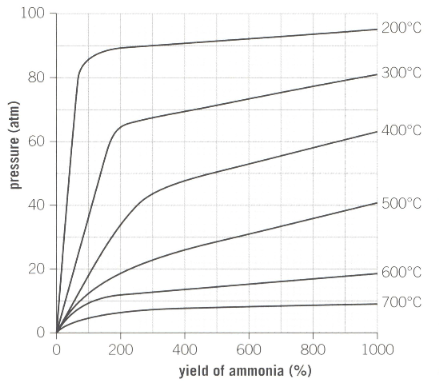
The ideal temperature used by Haber is approximately . Outline the reasons why a higher temperature would be used to increase production of ammonia instead of a lower temperature.
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
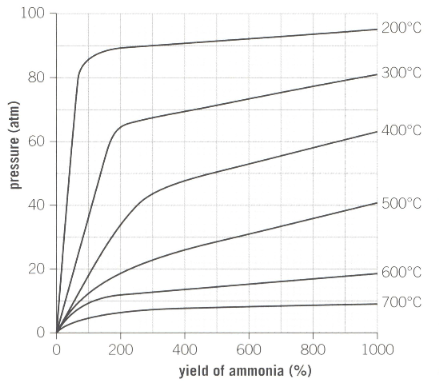
A pressure of is used during the process. Why does industry not use a much higher pressure to maximise yield?
Industrial reaction conditions for the production of ammonia is as shown below in the graph:
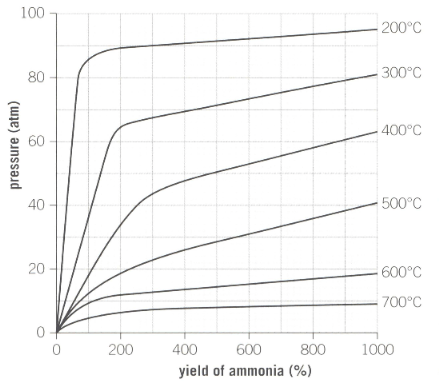
Explain with reference to the position of the equilibrium why increasing the pressure of this closed system favours the forward reaction.
