Photoelectric Effect and Wave Theory of Light
Important Questions on Photoelectric Effect and Wave Theory of Light
A particle with a mass is moving with a velocity and hits a particle (mass ) at rest (one dimensional motion). Find the change in the de Broglie wavelength of the particle . Treat the collision as elastic.
Consider a metal exposed to light of wavelength . The maximum energy of the electron doubles when light of wavelength is used. Find the work function in .
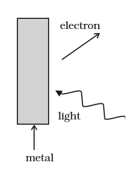
Consider figure for photoemission.
How would you reconcile with momentum-conservation? Note light (photons) have momentum in a different direction than the emitted electrons.
There are two sources of light, each emitting with a power of . One emits X-rays of wavelength and the other visible light at . Find the ratio of number of photons of X-rays to the photons of visible light of the given wavelength?
Do all the electrons that absorb a photon come out as photoelectrons?
There are materials which absorb photons of shorter wavelength and emit photons of longer wavelength. Can there be stable substances which absorb photons of larger wavelength and emit light of shorter wavelength.
A particle moves in a closed orbit around the origin, due to a force which is directed towards the origin. The de Broglie wavelength of the particle varies cyclically between two values , with . Which of the following statement are true?
Photons absorbed in matter are converted to heat. A source emitting photon per second of frequency is used to convert of ice at to water at . Then, the time taken for the conversion :
The de Broglie wavelength of a photon is twice the de Broglie wavelength of an electron. The speed of the electron is . Then
Two particles and of masses have the same de - Broglie wavelength. Then
Relativistic corrections become necessary when the expression for the kinetic energy , becomes comparable with , where is the mass of the particle. At what de Broglie wavelength will relativistic corrections become important for an electron?
An electron (mass m) with an initial velocity is in an electric field . If, , it's de-Borglie wavelength at time is given by
An electron (mass m) with an initial velocity , is in an electric field (constant ).It's de Broglie wavelength at time is given by
An electron is moving with an initial velocity and is in a magnetic field . Then it's de Broglie wavelength
A proton, a neutron, an electron and an -particle have same energy. Then their de Broglie wavelengths compare as
The wavelength of a photon needed to remove a proton from a nucleus which is bound to the nucleus with energy is nearly
A particle is dropped from a height . The de Broglie wavelength of the particle as a function of height is proportional to

