Physical Properties of Alcohols
Important Questions on Physical Properties of Alcohols
Give the IUPAC names of
Give the IUPAC names of the following compounds
Boiling points of alcohols are _____ than those of the corresponding alkanes.(less/more)
What is fermentation?
What is an enzyme? Name two enzymes used in the preparation of alcohol from sucrose.
Arrange the compounds to which have roughly the same molecular weight in order of decreasing boiling points.
(A)
(B)
(C)
(D)
Why ethanol has higher boiling point than
Why glycerol is more viscous than glycol?
Which out of the following has the highest boiling point?
(i)
(ii)
(iii)
(iv)
(v)
Arrange the following in the increasing order of boiling point
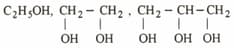
Arrange propan--ol, propan--ol and methoxyethane in the increasing order of their boiling points.
How would you account for the following:
The boiling points of ethers are much lower than those of the alcohols of comparable molar mass.
Account for the following :
The boiling points of ethers are lower than isomeric alcohols.
Account for the following :
The boiling point of ethanol is higher than that of methanol
Account for the following :
Propanol has higher boiling point than butane.
How is that alcohol and water are miscible in all proportions?
Phenol has smaller dipole moment than methanol. Why?
Which of the following will have higher boiling point and why? or
Why absolute ethyl alcohol cannot be obtained by simple fractional distillation of a mixture of alcohol and water?
Alcohols are comparatively more soluble than the hydrocarbons of comparable molecular masses. Explain.

