Law of Constant Proportions
Important Questions on Law of Constant Proportions
sample of a compound of oxygen and boron was found by analysis to contain of oxygen and of boron. Calculate the percentage composition of the compound by weight.
For all the molecules of water to be identical, it is essential that the atoms of hydrogen and oxygen that are present in the molecule must be in fixed numbers. If this number is not fixed, how could all the particles of water be identical?
Is it possible for any number of atoms of hydrogen to combine with any number of atoms of oxygen to form a molecule of water?
Discuss if the carbon dioxide that you breathe out and the carbon dioxide others breathe out is identical. Is the composition of the carbon dioxide of different sources the same?
of mercuric oxide decomposes to give of mercury and of oxygen. Let us assume that of oxygen reacts completely with of mercury to give mercuric oxide. Do these values agree with the law of constant proportions?
Proust took two samples of copper carbonate - a compound of copper, carbon and oxygen. He took a sample from nature and another sample prepared in the lab and decomposed it chemically to find the percentage of copper, carbon and oxygen in the two samples. The results obtained are given in the following table.
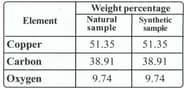
What difference do you observe in the percentage of copper, carbon and oxygen in the two samples?
Proust took two samples of copper carbonate - a compound of copper, carbon and oxygen. He took a sample from nature and another sample prepared in the lab and decomposed it chemically to find the percentage of copper, carbon and oxygen in the two samples. The results obtained are given in the following table.
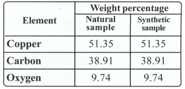
What do you observe from the table?

