Heat, Internal Energy and Work
Heat, Internal Energy and Work: Overview
In this topic, we will learn the concept of internal energy, heat and work. The concept of temperature from zeroth law is also explained here with the help of diagrams.
Important Questions on Heat, Internal Energy and Work
Explain why
(d) The climate of a harbour town is more temperate than that of a town in a desert at the same latitude.
A thermodynamic system is taken from an original state to an intermediate state by the linear process shown in the figure.
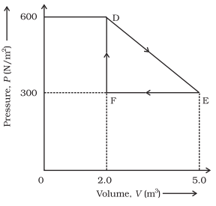
Its volume is then reduced to the original value from E to F by an isobaric process.
Calculate the total work done by the gas from D to E to F.
An electric heater supplies heat to a system at a rate of 100 W. If the system performs work at a rate of 75 joules per second, at what rate is the internal energy increasing?
A cylinder with a movable piston contains 3 moles of hydrogen at standard temperature and pressure. The walls of the cylinder are made of a heat insulator, and the piston is insulated by having a pile of sand on it. By what factor does the pressure of the increase if the gas is compressed to half its original volume?
