Pankaj Bhatt Solutions for Chapter: Calorimetry, Exercise 1: QUESTIONS
Pankaj Bhatt Physics Solutions for Exercise - Pankaj Bhatt Solutions for Chapter: Calorimetry, Exercise 1: QUESTIONS
Attempt the free practice questions on Chapter 11: Calorimetry, Exercise 1: QUESTIONS with hints and solutions to strengthen your understanding. S CHAND'S ICSE PHYSICS BOOK 2 solutions are prepared by Experienced Embibe Experts.
Questions from Pankaj Bhatt Solutions for Chapter: Calorimetry, Exercise 1: QUESTIONS with Hints & Solutions
of heat is given to of water at . What is the final temperature of water?
of water at is mixed with of water at . What is the final temperature of mixture?
of kerosene oil at is mixed with of water at . What is the temperature of the mixture? Specific heat of kerosene .
A small piece of iron of mass is heated to temperature and then put in a calorimeter of mass containing of water at . What is the final temperature of mixture? Specific heat of iron and of Calorimeter.
A solid of mass is heated to a temperature and then put in a calorimeter containing of kerosene oil at . If the mass of the calorimeter is what is the final temperature of the mixture? Specific heat of solid. Specific heat of kerosene oil.
A calorimeter of heat capacity contains of water at . A piece of metal of mass is heated up to and put in the calorimeter. The temperature of mixture is obtained . What is specific heat of metal?
A calorimeter of heat capacity contains of liquid at . A piece of metal of mass is heated up to and put in the calorimeter. The final temperature of mixture is obtained . If specific heat of metal is , calculate the specific heat of the liquid.
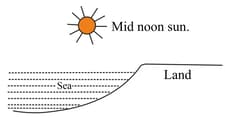
The figure given below shows a geographical phenomenon. Name this phenomenon and explain it in brief.