Law of Radioactive Decay
Important Questions on Law of Radioactive Decay
The count rate of a radioactive sample was at and at Select the correct alternatives
Radioactive nuclei are being generated at a constant rate by some kind of nuclear reaction. If the decay constant for the radioactive nuclei is , which of the following graphical representation is correct? (initially, there are no radioactive nuclei present)
The decay constant of a radioactive substance is . At , the number of active nuclei are . Select the correct alternative
Disintegration consicrit of a radioactive material is
In a sample of nuclei, 25 percent decay by -decay to and the rest decay by electron capture to . What is the ratio of the mean life for -decay, , to the mean life for electron capture,
Mean life of a radioactive substance is 1620 and 2160 years for and emission respectively. At any instant particles are emitted at a rate of and particles at Mass number of substance is and take time for year as If mass of radioactive substance at that instant is find (Take Avogadro no. ) (Approximate the answer to the nearest integer.)
Two radioactive nuclei and in a given sample decay into a stable nucleus . At time , the number of species are and that of are . Half-life of (for conversion to ) is whereas that of is . Initially, there are no nuclei of present in the sample. When number of nuclei of and are equal, the number of nuclei of present in the sample would be,
The activity of a radioactive substance decreased to one-third of the original activity number in a period of . After a further lapse of , its activity will be
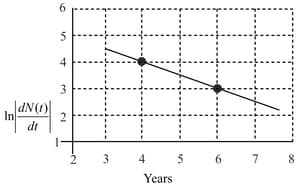
To determine the half life of a radioactive element, a student plots a graph of versus Here, is the rate of radioactive decay at time If the number of radioactive nuclei of this element decreases by a factor of after years, the value of is:
Nuclei of radioactive element are produced at rate at any time . The element has decay constant Let be the number of nuclei of element at any time At time is minimum. Then the number of nuclei of element at time is
A radioactive element converts into another stable element . Half life of is . Initially only is present. After time , the ratio of atoms of and is found to be , then in hours is
A sample of radioactive material decays simultaneously by two processes and with half lives and , respectively. For first half hour it decays with the process next one with the process and for further half an hour with both and . If originally there were nuclei, find the number of nuclei after of such decay.
A parent nucleus undergoes decay with a half-life of years. The daughter nucleus undergoes decay with a half-life of months. In a particular sample, it is found that the rate of emission of particles is nearly constant (over several months) at What will be the number of particles emitted in an hour?
A radioactive substance is being produced at a constant rate per second. Its decay constant is . The relation between the maximum number of nuclei of is , then the value of is
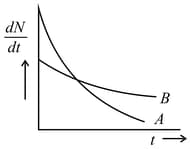
The variation of decay rate of two radioactive samples A and B with time is shown in figure. Which of the following statements are true?
fraction of a sample disintegrates in time. How much time it will take to disintegrate fraction?
A radioactive sample disintegrates by during one month. How much fraction will disintegrate in four months?
The decay constant of the parent nuclide in Uranium series is . Then, the decay constant of the stable end product of the series will be,
A freshly prepared radioactive source of half-life emits radiation of intensity which is times the permissible safe level. The minimum time after which it would be possible to work safely with this source is,
The activity of a sample of radioactive material is at time and at time . Its mean life is T. Which of the following is correct?

