Embibe Experts Solutions for Chapter: Dual Nature of Matter and Radiation, Exercise 2: Level 2
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Dual Nature of Matter and Radiation, Exercise 2: Level 2
Attempt the practice questions on Chapter 20: Dual Nature of Matter and Radiation, Exercise 2: Level 2 with hints and solutions to strengthen your understanding. Physics Crash Course JEE Main solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Dual Nature of Matter and Radiation, Exercise 2: Level 2 with Hints & Solutions
The de-Broglie wavelength of an electron is the same as that of a -ray photon. The ratio of the energy of the photon to the kinetic energy of the electron is (the energy equivalent of electron mass is )
The de-Broglie wavelength of an electron moving with a velocity (velocity of light in vacuum) is equal to the wavelength of a photon. The ratio of the kinetic energies of electron and photon is
The de-Broglie wavelength of an electron in the ground state of the hydrogen atom is
The threshold frequency for a certain metal is . If light of frequency is incident on the metal, then the cut-off voltage for the photoelectric emission is approximately is . The value of is
The work functions of silver and sodium are and , respectively. The ratio of the slope of the stopping potential versus frequency plot for silver to that of sodium is
The anode voltage of a photocell is kept fixed. The wavelength of the light falling on the cathode is gradually changed. The plate current of photocell varies as follows:
In a photoelectric experiment, with light of wavelength the fastest electron has speed If the exciting wavelength is changed to the speed of the fastest emitted electron will become
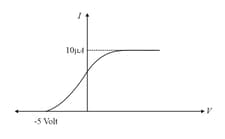
In the photoelectric experiment, if we use a monochromatic light, the curve is as shown. If work function of the metal is estimate the power of light used. (Assume efficiency of photo emission is i.e. number of photoelectrons emitted are of number of photons incident on metal.)