R.R. Mishra Solutions for Exercise 3: SUMMATIVE ASSESSMENT QUESTIONS (As Per the Guidelines of the CBSE on CCE)
R.R. Mishra Chemistry Solutions for Exercise - R.R. Mishra Solutions for Exercise 3: SUMMATIVE ASSESSMENT QUESTIONS (As Per the Guidelines of the CBSE on CCE)
Attempt the free practice questions from Exercise 3: SUMMATIVE ASSESSMENT QUESTIONS (As Per the Guidelines of the CBSE on CCE) with hints and solutions to strengthen your understanding. Secondary Chemistry Class X solutions are prepared by Experienced Embibe Experts.
Questions from R.R. Mishra Solutions for Exercise 3: SUMMATIVE ASSESSMENT QUESTIONS (As Per the Guidelines of the CBSE on CCE) with Hints & Solutions
Write a balanced chemical equation to describe the reaction between sodium metal and cold water. What would be observed when
(i) a burning candle is brought near the reaction mixture.
(ii) four drops of phenolphthalein are added in the reaction mixture.
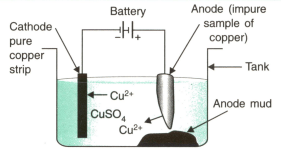
The given figure is the experimental setup to refine copper metal. Answer the following questions:
(i) Which material is used as anode?
(ii) In which direction do ions move in the electrolytic cell?
(iii) Name three impurities which collect as anode mud.
An ionic compound is formed between a metal and a nonmetal. Write two important physical properties of this compound.
Nonmetal A, a major gaseous component of air, combines with in the mole ratio in the presence of iron catalyst to give a gas B which is highly soluble in water. Gas A forms a reddish brown oxide C, When C is dissolved in water in the presence of air an acid D is formed which is a strong oxidizing agent.
(i) Identify A, B, C and D
(ii) What is the nature of the aqueous solution of gas B?
(iii) To which group and period of the period table does the nonmetal A belong?
Write the reactions involved in the following steps of the extraction of copper from its ore.
Roasting of .
Write the reactions involved in the following steps of the extraction of copper from its ore.
Reduction of withWrite the reactions involved in the following steps of the extraction of copper from its ore.
Electrolytic refining
Write the reactions involved in the following steps of the extraction of copper from its ore.
Draw a properly labelled diagram for electrolytic refining of copper.