Rajesh Singh, Indu Gupta and, Sikha Sharma Solutions for Chapter: Acids, Bases and Salts, Exercise 3: Long Answer Type Questions
Rajesh Singh Science Solutions for Exercise - Rajesh Singh, Indu Gupta and, Sikha Sharma Solutions for Chapter: Acids, Bases and Salts, Exercise 3: Long Answer Type Questions
Attempt the free practice questions on Chapter 2: Acids, Bases and Salts, Exercise 3: Long Answer Type Questions with hints and solutions to strengthen your understanding. NCERT EXEMPLAR PROBLEMS-SOLUTIONS SCIENCE Class X solutions are prepared by Experienced Embibe Experts.
Questions from Rajesh Singh, Indu Gupta and, Sikha Sharma Solutions for Chapter: Acids, Bases and Salts, Exercise 3: Long Answer Type Questions with Hints & Solutions
In the following schematic diagram for the preparation of hydrogen gas as shown in the figure, what would happen if the following changes are made?
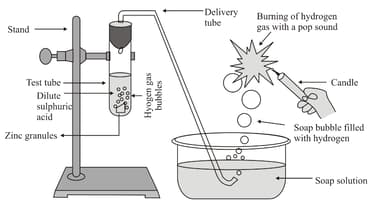
(a) In place of zinc granules, the same amount of zinc dust was taken in the test tube.
(b) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
(c) In place of zinc, copper turnings are taken.
(d) Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated.
For making cake, baking powder is taken. If at home mother uses baking soda instead of baking powder in cake.
(a) How will it affect the taste of the cake and why?
(b) How can baking soda be converted into baking powder?
(c) What is the role of tartaric acid added to baking soda?
A metal carbonate on reacting with an acid gives a gas which when passed through a solution gives the carbonate back. On the other hand, a gas that is obtained at anode during electrolysis of brine is passed on dry , it gives a compound , used for disinfecting drinking water. Identify and
A dry pellet of a common base , when kept in open absorbs moisture and turns sticky. The compound is also a by-product of chlor-alkali process. Identify , what type of reaction occurs when is treated with an acidic oxide. Write a balanced chemical equation for one such solution.
A sulphate salt of group 2 elements of the periodic table is a white, soft substance which can be moulded into different shapes by making its dough. When this compound is left in open for some time, it becomes a solid mass and cannot be used for moulding purpose. Identify the sulphate salt and why does it show such a behaviour? Given the reaction involved.
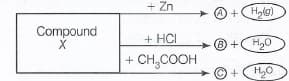
Identify the compound on the basis of the reactions given below. Also, Write the name and chemical formula of and .