Ramendra C Mukerjee Solutions for Chapter: Chemical Equilibrium, Exercise 2: Objective Problems
Ramendra C Mukerjee Chemistry Solutions for Exercise - Ramendra C Mukerjee Solutions for Chapter: Chemical Equilibrium, Exercise 2: Objective Problems
Attempt the practice questions on Chapter 15: Chemical Equilibrium, Exercise 2: Objective Problems with hints and solutions to strengthen your understanding. Modern Approach to Chemical Calculations solutions are prepared by Experienced Embibe Experts.
Questions from Ramendra C Mukerjee Solutions for Chapter: Chemical Equilibrium, Exercise 2: Objective Problems with Hints & Solutions
for is at . If a container contains and of and , respectively, at , the reaction shall
Which of the following curves represents a very rare standard reactivity at equilibrium?
Which of the curves given represents a standard reaction spontaneous in forward direction?
Which of the curves given represents a standard reaction for which
Which of the curves given represent a standard reaction with ?
The energy profile of the reaction, is shown as,
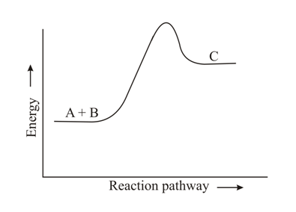
The equilibrium constant for the said equilibrium
is to be reduced to in an industrial process by the use of the reaction
At , the equilibrium constant for the reaction is . If a pressure of is to be employed in the furnace and the total pressure never exceeds will the reduction occur?
Which of the following curves between and is correct for an endothermic reaction?
