Ramendra C Mukerjee Solutions for Chapter: Chemical Kinetics, Exercise 2: Objective Problems
Ramendra C Mukerjee Chemistry Solutions for Exercise - Ramendra C Mukerjee Solutions for Chapter: Chemical Kinetics, Exercise 2: Objective Problems
Attempt the practice questions on Chapter 17: Chemical Kinetics, Exercise 2: Objective Problems with hints and solutions to strengthen your understanding. Modern Approach to Chemical Calculations solutions are prepared by Experienced Embibe Experts.
Questions from Ramendra C Mukerjee Solutions for Chapter: Chemical Kinetics, Exercise 2: Objective Problems with Hints & Solutions
Which of the following curves represents a first order reaction?
Which of the following curves represents a zero order reaction?
[reactant concentration]
Which of the following curves represent(s) a zero-order reaction?
For the chemical reaction of the type , the correct relationship amongst the rate expressions is:
For what type of the following reactions, is the law of mass action never obeyed?
If the rate law of a reaction is expressed as
The unit of the rate constant will be:
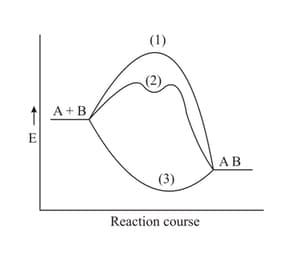
The exothermic reaction between substances and is presented in the plot below. Which pathway does a catalyst-induced preparation of the substance follow?
Which curve corresponds to the temperature dependence on the rate of a simple one-step reaction?
