Ramendra C Mukerjee Solutions for Chapter: Chemical Thermodynamics, Exercise 1: PROBLEMS
Ramendra C Mukerjee Chemistry Solutions for Exercise - Ramendra C Mukerjee Solutions for Chapter: Chemical Thermodynamics, Exercise 1: PROBLEMS
Attempt the practice questions on Chapter 14: Chemical Thermodynamics, Exercise 1: PROBLEMS with hints and solutions to strengthen your understanding. Modern Approach to Chemical Calculations solutions are prepared by Experienced Embibe Experts.
Questions from Ramendra C Mukerjee Solutions for Chapter: Chemical Thermodynamics, Exercise 1: PROBLEMS with Hints & Solutions
The heat of reaction of is . If the bond energies of and bonds are and , respectively, calculate the bond energy of bond.
Calculate the heat of formation of acetone from the following data:
Bond energies :
Calculate the heat of formation of methyl alcohol (liquid) from the following data:
Heat of atomisation of
Heat of atomisation of
Heat of atomisation of
Bond energies:
Heat of liquefaction of mole of .
Calculate the heat of the following gaseous reaction:
The bond energies of and bonds are and respectively.
Estimate the heat of formation of gaseous isoprene.
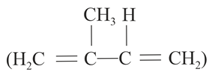
From the following data:
Bond energies:
Heat of sublimation of carbon(s) per mole.
Using the required bond-energies data given, calculate the heat of hydrogenation of ethene to ethane.
Bond energies:
Calculate the heat of the following homogeneous gaseous reaction
from the following data:
Bond energies :
Resonance energy:
Calculate the resonance energy of from the following data:
of
Bond energies of and bonds are and respectively.
