Richard Harwood and Ian Lodge Solutions for Chapter: Elements and Compounds, Exercise 4: Exercise 3.4
Richard Harwood Chemistry Solutions for Exercise - Richard Harwood and Ian Lodge Solutions for Chapter: Elements and Compounds, Exercise 4: Exercise 3.4
Attempt the free practice questions on Chapter 3: Elements and Compounds, Exercise 4: Exercise 3.4 with hints and solutions to strengthen your understanding. Cambridge IGCSE Chemistry Workbook 4th Edition solutions are prepared by Experienced Embibe Experts.
Questions from Richard Harwood and Ian Lodge Solutions for Chapter: Elements and Compounds, Exercise 4: Exercise 3.4 with Hints & Solutions
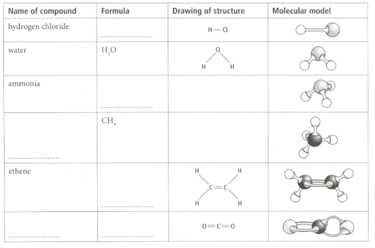
Many covalent compounds exist as simple molecules where the atoms are joined together with single or double bonds. A covalent bond, make up of a shared pair of electrons, is often represented by a short straight line.
Complete the table by filling in the spaces.
Graphite is one of the crystalline forms of carbon. Two of the distinctive properties of graphite are:
- It conducts electricity even though it is a non-metal, and
- It can act as a lubricant even though it has a giant covalent structure.
Give a brief explanation of these properties in light of the structure of graphite.
Graphite as an electrical conductor.
Graphite is one of the crystalline forms of carbon. Two of the distinctive properties of graphite are:
- It conducts electricity even though it is a non-metal, and
- It can act as a lubricant even though it has a giant covalent structure.
Give a brief explanation of these properties in light of the structure of graphite.
Graphite are as lubricant.