Richard Harwood and Ian Lodge Solutions for Chapter: How Far? How Fast, Exercise 2: Exercise 7.2
Richard Harwood Chemistry Solutions for Exercise - Richard Harwood and Ian Lodge Solutions for Chapter: How Far? How Fast, Exercise 2: Exercise 7.2
Attempt the practice questions on Chapter 7: How Far? How Fast, Exercise 2: Exercise 7.2 with hints and solutions to strengthen your understanding. Cambridge IGCSE Chemistry Workbook 4th Edition solutions are prepared by Experienced Embibe Experts.
Questions from Richard Harwood and Ian Lodge Solutions for Chapter: How Far? How Fast, Exercise 2: Exercise 7.2 with Hints & Solutions
The energy level diagram for an exothermic reaction is different from that for an endothermic reaction. The following keywords/phrases will be needed to fill in the information boxes accompanying the diagrams.
given out positive taken in reactants negative products
Exothermic reactions:
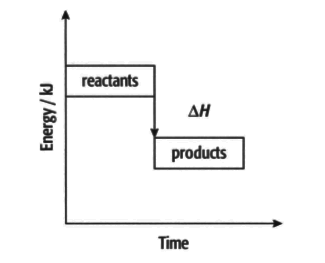
Use information from the diagram and the keywords/phrases to complete the following information.
In an exothermic reaction, the _____ have more energy than the _____. This means that is _____. The difference in energy is _____ as heat. The temperature of the surroundings increases/decreases. (Delete the incorrect word)
The energy level diagram for an exothermic reaction is different from that for an endothermic reaction. The following keywords/phrases will be needed to fill in the information boxes accompanying the diagrams.
given out positive taken in reactants negative products
Endothermic reactions:
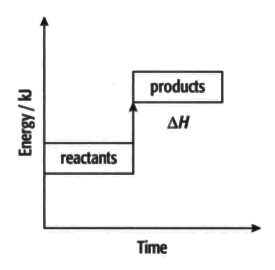
Use information from the diagram and the keywords/phrases to complete the following information.
In an endothermic reaction, the _____ have more energy than the _____. This means that is _____. The difference in energy is _____ as heat. The temperature of the surroundings increases/decreases. (Delete the incorrect word)
