RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Making Use of Metals, Exercise 7: Checkup on Chapter 14
RoseMarie Gallagher Chemistry Solutions for Exercise - RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Making Use of Metals, Exercise 7: Checkup on Chapter 14
Attempt the free practice questions on Chapter 14: Making Use of Metals, Exercise 7: Checkup on Chapter 14 with hints and solutions to strengthen your understanding. Complete Chemistry for Cambridge IGCSE® Second Edition solutions are prepared by Experienced Embibe Experts.
Questions from RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Making Use of Metals, Exercise 7: Checkup on Chapter 14 with Hints & Solutions
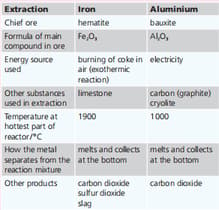
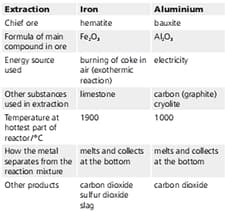
Earth’s crust. Iron is next. Iron and aluminium are extracted from their ores in large quantities. The table below summarises the two extraction processes. Explain why each of these substances is used: carbon in the extraction of aluminium.
| Extraction | Iron | Aluminium |
| Chief ore | hematite | bauxite |
| Formula of main compound in ore | ||
| Energy source used | burning of coke in air(exothermic reaction) | Electricity |
| Other substances used in extraction | limestone | carbon(graphite), cryolite |
| Temperature at hottest part of reaction/ | 1900 | 1000 |
| How the metal separates from the reaction mixture | melts and collects at the bottom | melts and collects at the bottom |
| Other products | Carbon dioxide, sulphur dioxide, slag | Carbon dioxide |
From Earth's crust; Iron and aluminium are extracted from their ores in large quantities. The table below summarises the two extraction processes. Explain why Cryolite is used in the extraction of aluminium.
| Extraction | Iron | Aluminium |
| Chief ore | hematite | bauxite |
| Formula of main compound in ore | ||
| Energy source used | burning of coke in air(exothermic reaction) | Electricity |
| Other substances used in extraction | limestone | carbon(graphite), cryolite |
| Temperature at hottest part of reaction/ | 1900 | 1000 |
| How the metal separates from the reaction mixture | melts and collects at the bottom | melts and collects at the bottom |
| Other products | Carbon dioxide, sulphur dioxide, slag | Carbon dioxide |
Earth’s crust. Iron is next. Iron and aluminium are extracted from their ores in large quantities. The table below summarises the two extraction processes. Describe any two similarities in the extraction.
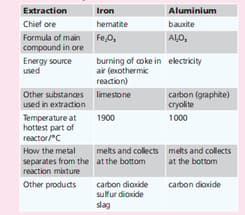
Aluminium is the most abundant metal in the Earth’s crust. Iron is next. Iron and aluminium are extracted from their ores in large quantities. The summarises the two extraction processes.Give a use for the slag that is produced as a by-product in the extraction of iron.
| Extraction | Iron | Aluminium |
| Chief ore | hematite | bauxite |
| Formula of main compound in ore | ||
| Energy source used | burning of coke in air(exothermic reaction) | Electricity |
| Other substances used in extraction | limestone | carbon(graphite), cryolite |
| Temperature at hottest part of reaction/ | 1900 | 1000 |
| How the metal separates from the reaction mixture | melts and collects at the bottom | melts and collects at the bottom |
| Other products | Carbon dioxide, sulphur dioxide, slag | Carbon dioxide |
Aluminium is the most abundant metal in the Earth’s crust. Iron is next. Iron and aluminium are extracted from their ores in large quantities. The tablebelow summarises the two extraction processes. Aluminium costs over three times as much per tonne as iron. Suggest two reasons why aluminium is more expensive than iron, even though it is more abundant in the Earth’s crust.
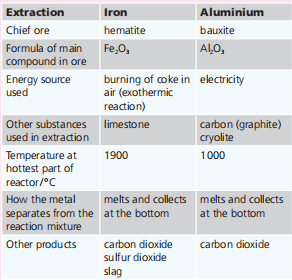
Aluminium is the most abundant metal in the Earth’s crust. Iron is next. Iron and aluminium are extracted from their ores in large quantities. The table below summarises the two extraction processes. Most of the iron produced is converted into steels. Why?
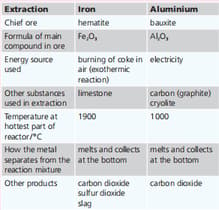
Aluminium is the most abundant metal in the Earth’s crust. Iron is next. Iron and aluminium are extracted from their ores in large quantities. The tablebelow summarises the two extraction processes.Most of the iron produced is converted into steels. How is this carried out?
Aluminium is the most abundant metal in the Earth’s crust. Iron is next. Iron and aluminium are extracted from their ores in large quantities. The table below summarises the two extraction processes.Both steel and aluminium are recycled. Suggest reasons why it is important to recycle these metals